Abstract
Bone mass measurement (BMM) is useful to identify persons with low bone mass who are at increased risk for fracture. Given the increased emphasis that is being placed on preventive services such as screening for osteoporosis, we evaluated trends in BMM among Medicare beneficiaries. We studied a 5% sample of Medicare beneficiaries ≥65 yr of age in 1999–2005. We identified claims for BMM tests performed in both facility and nonfacility settings, evaluated temporal trends in use of these tests, and described the proportion of tests attributable to each specialty of physicians submitting claims. We also assessed patterns of serial testing among individuals who were tested more than once. Claims data from all years were pooled to describe the proportion of persons in the population ever tested. From 1999 to 2005, use of central DXA increased by ∼50%, and use of peripheral DXA declined. The greatest increases in central DXA occurred among internists, family practitioners, and gynecologists. In 1999, the proportion of 65-yr-old women tested was 8.4%; this increased to 12.9% in 2005. Corresponding proportions for men were 0.6% and 1.7%, respectively. Between 40% and 73% of persons receiving central DXA were retested, most at ∼2-yr intervals. Aggregating data across all years for whites and blacks, 30.0% of women and 4.4% of men underwent central DXA at least once. We conclude that, although use of DXA steadily increased from 1999 to 2005, only ∼30% of women and 4% of men at least 65 yr old had a central DXA study. Given the importance of central DXA to assess the risk of osteoporotic fractures, strategies to increase central DXA use to test at-risk persons are warranted.
Key words: osteoporosis, BMD, epidemiology, DXA
INTRODUCTION
Bone mass measurement (BMM) is a well-validated and widely accepted screening test to identify patients with low bone mass who are at increased risk for fragility fractures. Because osteoporosis is clinically asymptomatic until a fracture occurs, the importance of screening during the asymptomatic phase is critical to identify opportunities to mitigate risk.(1) There are many types of BMM testing technologies, including ultrasound, QCT, and both single- and dual-energy X-ray absorptiometry. Among these, DXA of central sites (lumbar spine, femoral neck, total hip) is preferred because of its precision, minimal radiation exposure, relatively low cost, and largest evidence base to support diagnostic and treatment guidelines.
Numerous international agencies recommend primary screening with DXA for at-risk persons. In the United States, the National Osteoporosis Foundation and the U.S. Preventive Task Force recommend population-wide DXA for all women at least 65 yr old and for younger women with risk factors.(2–4) Screening with central DXA for all women ≥65 yr of age also is recommended by Medicare as one of the reimbursable quality measures that is part of the 2007 Physician Quality Reporting Initiative (PQRI). There is less consensus on the appropriate screening age for men at average risk, although the International Society for Clinical Densitometry recommends screening for men beginning at age 70. Screening with DXA has been shown to mediate use of prescription therapies for osteoporosis(5) and to reduce fracture-related morbidity.(6) Greater awareness of the public health burden of osteoporosis and the benefits of screening with DXA have assumed increased prominence in the United States in recent years and are an important focus of the U.S. Surgeon General's Report on Bone Health.(7)
The Bone Mass Measurement Act was passed by Congress in 1997 and provides DXA reimbursement for qualified Medicare beneficiaries in five categories. These five indications are primary preventive screening for estrogen-deficient women, and indications for both sexes include long-term glucocorticoid therapy, hyperparathyroidism, vertebral abnormalities, and longitudinal assessment of response to approved osteoporosis medications. The “Welcome to Medicare Exam” now includes BMM as a basic service. Most U.S. commercial insurance carriers have adopted coverage guidelines similar to Medicare. Legislation passed in 2007 has reduced reimbursement for outpatient DXA for Medicare enrollees by 40%, with additional cuts expected in future years.
In light of these considerations and to compare with potential future changes in DXA use, we evaluated trends in BMM use from 1999 to 2005 (before reimbursement cuts were enacted) among older Americans. As part of this study, we evaluated changes in DXA use across physician specialties and described patterns of serial DXA testing.
MATERIALS AND METHODS
Medicare data source and eligible population
After approval of the study protocol by the University of Alabama at Birmingham institutional review board and the Center for Medicare and Medicaid Services (CMS), we obtained research-identifiable files containing demographic information on individual beneficiaries and Medicare inpatient, outpatient, and physician claims data for 1999–2005 for persons randomly selected for inclusion in the Medicare 5% sample. Beneficiary identification number was used to link data across files. Except for particular subanalyses where exceptions are specifically mentioned, subjects were Medicare beneficiaries ≥65 yr of age living in the 50 United States and the District of Columbia. For each year, subjects had 12 mo of fee-for-service (FFS) Medicare part A and part B and were not enrolled in a health maintenance organization (HMO). Persons receiving care from a Medicare HMO for part or all of a year were excluded because Medicare data do not include all of their outpatient claims. Although some Medicare beneficiaries are <65 yr of age, most of these individuals are covered by Medicare because of disability or end-stage renal disease. We excluded these people because their DXA use is likely to differ from that of the general population of Medicare beneficiaries. In accordance with Medicare policies, use of the Medicare data was governed by a Data Use Agreement, and all results were reviewed by CMS before public release.
Identification of BMM testing
To identify claims submitted to Medicare for BMM for 1999–2005, we used Healthcare Current Procedure Classification System (HCPCS) codes and International Classification of Disease, 9th revision (ICD-9) procedure codes in claims from the Medicare carrier and outpatient files. HCPCS procedure codes included 76075 (central DXA) and 76076 (peripheral DXA). We also defined a group of other BMM technologies using HCPCS procedure codes 76070 (CT BMD study, one or more sites; axial skeleton [e.g., hips, pelvis, spine]), 76078 (radiographic absorptiometry [e.g., photodensitometry, radiogammetry] one or more sites), 78350 (BMD [BMC] study, one or more sites; single-photon absorptiometry), 78351 (BMD [BMC] study, one or more sites; dual-photon absorptiometry), 76977 (ultrasound BMD measurement and interpretation, peripheral site(s), any method), and the ICD-9 procedure code 88.98. BMM procedures are typically billed either as a single claim, indicating that the billing provider (e.g., a physician) both performed and interpreted the test in an office-based setting, or alternatively, as two claims, one for the technical charge for the test and another for interpretation. In the latter circumstance, a testing facility (e.g., the outpatient department of a hospital) usually bills for the technical charge, and a physician bills for the interpretation. Because claims for the technical charge and the interpretation are often not submitted by the same provider or on the same day, the use of HCPCS modifiers -TC (technical component only) and -26 (professional component only) were examined to identify facility claims and link these separate components. Claims for BMM occurring with 15 days of one another were aggregated together as a single unit to permit such linkage.
Data analysis
After identifying the number and types of BMMs testing in each year, results from the 5% sample were multiplied by 20 to obtain estimates of the total number of BMMs performed for the entire Medicare FFS population. Because the majority of U.S. diagnosis and treatment guidelines based on BMM results are specific to central DXA, further analyses focused exclusively on the use of this test (HCPCS code 76075).
To describe the specialty of the providers ordering DXA, the specialty of the service provider submitting the claim for each DXA was used as a surrogate. A field identifying specialty is available in Medicare data and is unique to each claim. However, some DXAs performed in outpatient departments identify the testing facility and the radiologist interpreting the test as the specialty of the providers. In this case, these specialties are not appropriate surrogates for the specialty of the physician who actually ordered the test. Therefore, these facility claims were excluded from the specialty-specific calculations for this particular analysis.
Because DXA can be ordered either as initial screening for patients with possible low bone mass or to monitor serial changes in BMD, we wished to differentiate these indications. Indeed, in December 2006, CMS published modifications to the BMM Act of 1997 so that central DXA would be the only BMM technology that would be reimbursed to monitor the response to drug therapy. To evaluate patterns of serial testing with DXAs, we identified tests performed in each calendar year and reported the proportion of people receiving DXA in that year who did and did not have a repeat DXA in any subsequent year. We also evaluated the interval of time between successive DXAs among those who were retested. Because our expectation was that repeat testing would occur for most patients at 2- to 3-yr intervals, the length of the gap between serial DXAs was evaluated only for pairs of DXAs ordered where the first DXA of a pair occurred any time in 1999–2002. Repeat DXAs were identified any time from 1999 to 2005. To describe trends in DXA use where testing might represent initial screening, we identified persons who were exactly 65 yr old in each calendar year from 1999 to 2005. The proportion of people tested in each year was calculated in separate sex strata. The change in the proportion tested across calendar years was evaluated to see if the rate of DXA screening increased over time.
DXA is not a service that typically needs to be repeated yearly, although it may be useful in situations where substantial bone loss is expected over short periods of time, such as among persons using long-term, high-dose glucocorticoid therapy. For persons without these conditions and if bone mass is normal, physicians may not feel that a repeat DXA is indicated for several years or more. For that reason, if a claims data source is used to evaluate the proportion of a population ever tested and if there are an insufficient number of longitudinal years of claims data available (or if the median individual follow-up time available in the claims data are relatively short), the proportion of the population tested is likely to be underestimated. To evaluate this possibility, we determined the proportion of eligible Medicare enrollees in 2005 with complete FFS coverage from 1999 to 2005 who had ever had one or more DXAs. Data were stratified by sex and white versus black race. To evaluate the amount of information bias that would result if we did not have the full 7 yr of claims data for each person, we performed separate analyses to simulate the effect on our results if we had fewer years of data available. Data were stratified by the number of consecutive years of claims data used to estimate the proportion of persons ever tested.
Finally, we recognize that, although 97% of all Americans ≥65 yr of age are covered by Medicare,(8) there are a minority of individuals in this age group with other types of insurance. The most common form of insurance coverage for non-Medicare enrollees in this age group is private insurance obtained through an employer. To establish that our results were generalizable to persons with private insurance, we examined receipt of BMD testing in 2005 among persons ≥65 yr of age with private insurance compared with those with Medicare. For this analysis, we used non–research-identifiable data from the 2005 public use files of the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey (NHAMCS). NAMCS and NHAMCS are annual, federally sponsored surveys that collect data provided by randomly selected physicians regarding patient encounters that occur in office-based settings or in outpatient departments associated with nonfederal hospitals. Beginning in 2005, BMD testing ordered by the health care provider is collected for each encounter as a specifically mentioned component of the one-page NAMCS and NHAMCS data collection form. NAMCS and NHAMCS provides weights that account for the complex sampling design and provide generalizability of results to all outpatient encounters occurring in nongovernmental settings in the United States.
Statistical methods
Descriptive statistics were used for all comparisons, and trend tests compared longitudinal changes in the proportion receiving DXA testing across years. SAS 9.1 (SAS Institute, Cary, NC, USA) was used for data management and all analyses. SAS-callable SUDAAN was used for analysis of NAMCS/NHAMCS data. The SUDAAN procedure PROC RLOGISTIC evaluated the relationship between receipt of BMD testing and private insurance (referent to Medicare). Crude models derived from NAMCS/NHAMCS data were subsequently adjusted for ethnicity, age, sex, number of medications, total number of comorbidities specifically queried on the survey instrument (range, 0–14), and the use of any drugs approved by the FDA for the prevention or treatment of osteoporosis (i.e., alendronate, risedronate, ibandronate, raloxifene, teriparatide, and calcitonin). Systemic estrogens were not included in this group because they may be used for indications other than to prevent or treat low bone mass.
RESULTS
The characteristics of Medicare beneficiaries ≥65 yr of age in 2005 with 12 mo of Medicare part A and part B coverage not enrolled in an HMO residing in the 50 United States that were used for subsequent analyses are shown in Table 1. A majority of persons were white, and there were more women than men in the sample. Approximately three quarters of beneficiaries lived in metropolitan areas. The number and types of BMM tests performed in each year from 1999 through 2005 is shown in Table 2. The number of central DXAs increased ∼50% during this period, and the use of peripheral DXA declined. Approximately two thirds of central DXAs were performed in office-based settings, and the largest increase in the number of central DXAs performed occurred from 1999 to 2002. Because the remainder of the analyses focus on the use of central DXA, all subsequent use of the term DXA refers to central, and not peripheral, DXA.
Table 1.
Characteristics of Medicare Cohort Eligible* in 2005 to Receive DXA
| Beneficiaries (%) | |
| Total | 25,783,720 (100) |
| Race/ethnicity | |
| Black | 1,966,800 (7.6) |
| White | 22,654,520 (87.9) |
| Other | 1,162,400 (4.5) |
| Sex | |
| Female | 15,142,440 (58.7) |
| Male | 10,641,280 (41.3) |
| Age, overall (yr) | |
| 65–69 | 5,954,300 (23.1) |
| 70–74 | 6,215,020 (24.1) |
| 75–79 | 5,552,700 (21.5) |
| 80+ | 8,061,700 (31.3) |
| Region | |
| Northeast | 4,948,200 (19.2) |
| Midwest | 6,672,020 (25.9) |
| West | 4,211,600 (16.3) |
| South | 9,951,900 (38.6) |
| Location | |
| Metropolitan | 18,816,800 (73.0) |
| Rural | 6,966,920 (27.0) |
*Eligible persons must have had 12 mo of Medicare Part A + Part B and be ≥65 yr of age at the end of 2005. These national estimates reflect data from the Medicare 5% sample.
Table 2.
Number of BMM Tests Ordered 1999–2005, by Type of Test and Location, Among Eligible* Medicare Beneficiaries
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | |
| Central DXAs | |||||||
| Nonfacility† | 678,960 | 771,600 | 842,160 | 922,160 | 947,280 | 978,640 | 999,020 |
| Facility† | 279,560 | 346,760 | 385,900 | 421,220 | 439,620 | 428,840 | 437,640 |
| Total | 958,520 | 1,118,360 | 1,228,060 | 1,343,380 | 1,386,900 | 1,407,480 | 1,436,660 |
| Peripheral DXAs | |||||||
| Nonfacility† | 72,040 | 54,740 | 43,240 | 38,220 | 30,980 | 27,200 | 21,000 |
| Facility† | 14,840 | 16,000 | 10,240 | 7,100 | 5,420 | 4,260 | 5,560 |
| Total | 86,880 | 70,740 | 53,480 | 45,320 | 36,400 | 31,460 | 26,560 |
| Any other BMM‡ | 42,540 | 37,780 | 37,980 | 32,980 | 60,120 | 55,900 | 54,660 |
* Eligible persons must have had 12 mo of Medicare Part A + Part B and be ≥65 yr of age at the end of the year in which the test was performed. These national estimates reflect data from the Medicare 5% sample.
† Nonfacility claims are those performed in settings such as a physician office. Facility claims are those performed in settings such as in the hospital or the outpatient radiology department of a hospital.
‡Other BMM includes QCT, single- and dual-photon absorptiometry, and ultrasound BMD measurements.
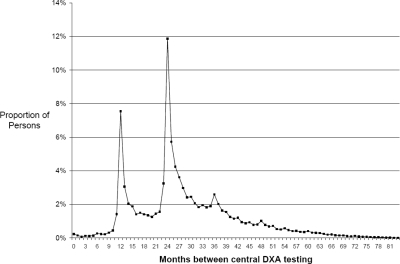
The proportion of persons who were tested with central DXA in 1999–2002 who had at least one subsequent DXA through 2005 varied substantially by the number of years of follow-up time. For DXAs performed in each year from 1999 through 2002, the proportions of people with any repeat DXA were 73%, 65%, 53%, and 40%, respectively. Among those who received at least two tests, the mean and median intervals between successive DXAs were 2.4 and 2.2 yr, and the pattern of the intervals between serial DXAs is shown in Fig. 1. Approximately 31% of persons with repeat DXAs were tested at intervals <2 yr apart, and there were modal peaks at 1 and 2 yr. The size of the peak at 1 yr was smaller in states where the local Medicare carrier did not routinely pay for serial testing at intervals <2 yr (data not shown). Among all pairs of serial DXAs where the second DXA was performed <2 yr after the first, Medicare did not pay for at least one of the tests in one third of the pairs.
FIG. 1.
Distribution of gaps between successive central DXAs for individuals having >1 DXA.* * Eligible persons must have had 12 mo of Medicare Part A + Part B each year from 1999 to 2005, be ≥65 yr of age at the end of 1999, and be in the Medicare 5% sample. Intervals between successive DXAs are shown only where the initial test was ordered in 1999–2002 given the need to allow for sufficient follow-up time to evaluate serial testing patterns.
The specialties of the physicians submitting claims for DXA in 1999 and 2005 are shown in Table 3. Approximately one half of all central DXAs were performed by internists, family practitioners, and gynecologists. Rheumatologists, endocrinologists, and orthopedists accounted for a majority of the remainder. Although all physician specialties increased their use of DXAs, the greatest increases were among primary care physicians: internists, family practitioners, and gynecologists.
Table 3.
Number and Distribution of Specialties* of Providers Submitting Claims for Central DXA Performed in Nonfacility Settings in 1999 and 2005†
| Physician specialty |
1999 |
2005 |
Absolute change | Relative increase‡ (%) | ||
| N | Percent | N | Percent | |||
| Internal medicine | 132,540 | 33.4 | 243,540 | 40.0 | 111,000 | 84 |
| Family/general practice | 52,340 | 13.2 | 95,380 | 15.7 | 43,040 | 82 |
| Rheumatology | 89,460 | 22.5 | 105,700 | 17.4 | 16,240 | 18 |
| Obstetrics/gynecology | 34,980 | 8.8 | 50,900 | 8.4 | 15,920 | 46 |
| Endocrinology | 33,300 | 8.4 | 44,580 | 7.3 | 11,280 | 34 |
| Orthopedic surgery | 26,720 | 6.7 | 27,320 | 4.5 | 600 | 2 |
| Other specialties | 27,680 | 7.0 | 41,800 | 6.9 | 14,120 | 51 |
| Total | 397,020 | 100.0 | 609,220 | 100.0 | 212,200 | 53 |
* Excludes claims submitted by radiologists or where physician specialty was not identified.
† Eligible persons must have had full year Medicare Part A + Part B, be ≥65 yr of age, at the end of the respective year, and be in the Medicare 5% sample.
‡ Reflects the absolute change in the number of DXAs performed (column 6) divided by the number performed in 1999 (column 2).
Table 4 shows the longitudinal trends in the proportion tested among persons age 65, stratified by sex. There were significant differences at specific years in the proportion of both men and women who received a DXA. The proportion tested increased across years for women but not for men. Table 5 shows the proportion of persons eligible in 2005 who were ever tested in the 7-yr observation period. Using data from all 7 yr, the proportion ever tested was 31.3% for white women compared with 15.3% for black women (p < 0.0001). For men, the respective proportions were 4.6% and 1.9% (p < 0.0001). Combining data from the two racial/ethnic groups across all available years, the total proportion of women and men who were tested was 30.0% and 4.4%, respectively. Restricting the analysis to fewer years of data showed that estimates of the proportion of eligible persons tested varied dramatically by the amount of information available. For example, if only 2 yr of claims data were used, the estimated proportion of persons tested was underestimated by ∼50%. After ∼3–4 yr of data were used, the trend seemed to plateau.
Table 4.
Increase in the Annual Rate of DXA Use Over 1999–2005 Among Eligible* Persons, Stratified by Sex
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | p for trend | |
| Women | 8.4 | 8.8 | 9.8 | 10.1 | 11.2 | 12.3 | 12.9 | 0.0001 |
| Men | 0.6 | 0.5 | 1.6 | 1.3 | 1.5 | 2.1 | 1.7 | 0.017 |
* Eligible persons must have had 12 mo of Medicare Part A + Part B, be exactly age 65 at the beginning of the year, and be in the Medicare 5% sample.
Table 5.
Proportion of Eligible* Persons Ever Tested With Central DXA, by Sex and Race and Stratified by the Number of Years of Claims Data Used
|
Women (%) |
Men (%) |
|||
| White (n = 13,912,780) | Black (n = 1,229,660) | White (n = 9,904,140) | Black (n = 737,140) | |
| 2005 data only | 8.2 | 4.1 | 1.4 | 0.6 |
| +2004 data | 15.5 | 7.7 | 2.4 | 1.0 |
| +2003 data | 20.7 | 10.4 | 3.2 | 1.4 |
| +2002 data | 24.5 | 12.3 | 3.7 | 1.6 |
| +2001 data | 27.4 | 13.6 | 4.1 | 1.7 |
| +2000 data 30.0 | 30.0 | 14.6 | 4.4 | 1.8 |
| +1999 data (i.e., all data used) | 31.3 | 15.3 | 4.6 | 1.9 |
* Eligible persons must have had full year Medicare Part A + Part B from 1999 to 2005, be ≥65 yr of age at the end of 1999, and be in the Medicare 5% sample.
Finally, combined data from NAMCS and NHAMCS were used to evaluate whether persons ≥65 yr of age with private insurance were more or less likely to receive DXA compared with individuals with Medicare. After adjusting for a number of factors hypothesized to be associated with BMD testing including age, sex, race, number of medications, total number of comorbidities, and filling a prescription for an osteoporosis medication, obtaining a DXA study was similar among persons covered by private insurance compared with persons covered by Medicare (adjusted OR = 1.2; 95% CI = 0.5–3.0; p = 0.69).
DISCUSSION
Using longitudinal claims data on Medicare beneficiaries from 1999 to 2005, we observed that the number of central DXA claims increased 50% during this time period, with the greatest increases in use occurring among internists and family practitioners. In 2005, 70% of all CMS claims for DXA studies came from the nonfacility (private practice) setting. The proportion tested among 65-yr-old women steadily increased over time and remained stable for men. Approximately 40–70% of persons who were tested with central DXA were retested, most 1 or 2 yr later. Using data from all 7 yr, 30% of eligible female Medicare beneficiaries had a central DXA study compared with only 4% of eligible men, and use of fewer years of information substantially underestimated the proportion of persons in the population ever tested.
Our estimates of the total number of BMM tests are lower than those reported in an earlier study that quantified the number of tests performed each year among Medicare enrollees ≥65 yr of age.(9) In that study, there were an estimated 1.7 million BMM tests performed in 2000, which exceeds our estimate of ∼1.2 million tests. This difference likely reflects our exclusion of persons who had part or full year enrollment in a Medicare HMO or who died part way through the observation period. The 13% rate of increase in the use of BMM tests from 1999–2000 cited in the earlier study is similar to our 1-yr rate of change of 16%. Highlighting the importance of our longitudinal, multiyear data, the earlier study estimated that the proportion of women tested was 8.8%; this estimate is similar to our estimate based on only 1 yr of data but is considerably lower than our estimate of 30% based on our multiyear results. This suggests that, particularly for white women, although rates of DXA testing are still suboptimal, approximately one third of older women have been tested within the previous several years, and physicians have more information available for clinical decision-making than prior estimates based on fewer years of longitudinal data might suggest. However, use of DXA is much lower than use of other screening services such as mammography, where ∼66% of women have been tested within the past 2 yr.(10) BMM use rates among men were much lower (4%), which may reflect the fact that the BMM Act of 1997 does not permit testing in men unless they have a vertebral fracture, known osteoporosis, steroid use, or hyperparathyroidism. Given that prior work has shown that access to DXA significantly mediates use of prescription osteoporosis therapies,(5) suboptimal use rates of DXA can be expected to associate with low use of medications to prevent fractures.
In light of the U.S. Preventive Services Task Force recommendations that all women ≥65 yr of age receive a BMD test, and the Surgeon General's report that emphasizes the importance of prevention and treatment of osteoporosis, the overall low use rates of DXA we observed are concerning. Additional research into factors about why patients have failed to receive DXA may be useful; however, it is likely that there is no dominant factor that is singly responsible that will be identified. Factors related to patients, providers, and the environments in which care is provided are likely to all be important and need to be addressed to increase rates of DXA use. Osteoporosis quality improvement interventions that principally target only the physician are likely to have only a modest impact, at best.(11) Systems-based interventions such as allowing self-referral of at-risk persons, as has been shown to be successful for mammography,(12) may be useful to increase DXA use rates. Developing customized, patient-directed interventions that consider factors described in the Health Belief Model, perhaps in conjunction with absolute fracture risk estimates, may provide added benefit. Additionally, scaling back recent cuts in DXA reimbursement would also be anticipated to preserve access to this service. There were >2 million fractures in the United States in 2005, resulting in a cost of 17 billion dollars.(13) In light of the 50% expected increase in fractures and associated costs over the next 20 yr, the public health implications of inadequate prevention efforts are substantial.
The strengths of our work include use of 7 yr of Medicare claims data, which allowed us to not only evaluate year-by-year changes in overall DXA use but also to link longitudinal data specific to individual persons. We aggregated data not only from the physician (carrier) billing files but also from outpatient departments, which allowed us to identify tests performed where there was no physician claim for interpretation. This latter circumstance might occur in settings where the physician is an employee of the hospital, and it would result in under-ascertainment of DXA use if only the carrier (physician) files were available. Additionally, the availability of longitudinal data allowed us to evaluate patterns of serial testing across years to determine the proportion of persons retested with central DXA and also the intervals between testing.
Despite these strengths, the limitations of our study must be considered. First, with respect to the analysis of physician specialties, it is possible that primary care physicians refer patients to receive DXA in the offices of specialists (i.e., a nonfacility setting), so the proportion of specialists is overrepresented. However, despite this possibility, we still showed that the greatest increase in central DXA use was attributable to primary care physicians. Second, we evaluated DXA testing among persons exactly 65 yr of age in an effort to identify persons newly tested. Despite showing increasing trends in the proportion receiving DXAs, particularly for women, we recognize that they may have been previously tested at a younger age when they had another form of insurance. This possibility would likely attenuate the rate of increase in testing over time. Finally, although Medicare claims data have the ability to evaluate serial patterns of use of BMM services, we lacked information on the results of those tests or the subsequent use of osteoporosis medications for persons tested, which have been shown to substantially increase over time.(14) It is likely that these factors were important determinants of the perceived need by physicians for retesting their patients with DXA, but test results and medication use were not available in this data source.
In conclusion, we observed that the use of BMM services increased substantially since 1999. The greatest increases were observed in the use of central DXA, particularly by primary care physicians. Thirty percent of women with Medicare who were ≥65 yr of age had been tested at least once from 1999 to 2005, and ∼5% of men had ever been tested. Although trends showed that rates of testing were increasing over time, the impact of recent legislation and regulatory changes that significantly decreased reimbursement of central DXA performed in nonfacility settings for Medicare beneficiaries has yet to be determined. Quality improvement interventions that simultaneously target patient-, provider-, and systems-related factors to increase DXA use continue to be needed.
ACKNOWLEDGMENTS
This research was supported by a contract between UAB and Amgen. Only the authors from UAB had access to the Medicare data used. The analysis, presentation, and interpretation of the results were solely the responsibility of the authors. Some of the investigators (JRC, KGS) also receive salary support from the National Institutes of Health (AR053351, AR052361), the Agency for Healthcare Research and Quality (U18 HS016956), and the Arthritis Foundation (JRC). Funding to support the analyses of NAMCS/NHAMCS data was provided through an unrestricted research grant to JRC by Proctor & Gamble.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529–541. doi: 10.7326/0003-4819-137-6-200209170-00015. [DOI] [PubMed] [Google Scholar]
- 3.International Society for Clinical Densitometry. Position statement: Executive summary. The Writing Group for the International Society for Clinical Densitometry (ISCD) Position Development Conference. J Clin Densitom. 2004;7:7–12. doi: 10.1385/jcd:7:1:7. [DOI] [PubMed] [Google Scholar]
- 4.National Osteoporosis Foundation. Belle, Mead, NJ, USA: Excerpta Medica; 1998. Physician's Guide to Prevention and Treatment of Osteoporosis. [Google Scholar]
- 5.Cadarette SM, Gignac MA, Jaglal SB, Beaton DE, Hawker GA. Access to osteoporosis treatment is critically linked to access to dual-energy x-ray absorptiometry testing. Med Care. 2007;45:896–901. doi: 10.1097/MLR.0b013e318054689f. [DOI] [PubMed] [Google Scholar]
- 6.Kern LM, Powe NR, Levine MA, Fitzpatrick AL, Harris TB, Robbins J, Fried LP. Association between screening for osteoporosis and the incidence of hip fracture. Ann Intern Med. 2005;142:173–181. doi: 10.7326/0003-4819-142-3-200502010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Bone Health and Osteoporosis. Rockville, MD, USA: U.S. Dept of Health and Human Services; 2004. A Report of the Surgeon General. [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.King AB, Saag KG, Burge RT, Pisu M, Goel N. Fracture Reduction Affects Medicare Economics (FRAME): Impact of increased osteoporosis diagnosis and treatment. Osteoporos Int. 2005;16:1545–1557. doi: 10.1007/s00198-005-1869-5. [DOI] [PubMed] [Google Scholar]
- 10.Breen N, Cronin KA, Meissner HI, Taplin SH, Tangka FK, Tiro JA, McNeel TS. Reported drop in mammography: Is this cause for concern. Cancer. 2007;109:2405–2409. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 11.Curtis JR, Westfall AO, Allison J, Becker A, Melton ME, Freeman A, Kiefe CI, MacArthur M, Ockershausen T, Stewart E, Weissman N, Saag KG. Challenges in improving the quality of osteoporosis care for long-term glucocorticoid users: A prospective randomized trial. Arch Intern Med. 2007;167:591–596. doi: 10.1001/archinte.167.6.591. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry R, Scheitel SM, McMurtry EK, Leutink DJ, Cabanela RL, Naessens JM, Rahman AS, Davis LA, Stroebel RJ. Web-based proactive system to improve breast cancer screening: A randomized controlled trial. Arch Intern Med. 2007;167:606–611. doi: 10.1001/archinte.167.6.606. [DOI] [PubMed] [Google Scholar]
- 13.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 14.Stafford RS, Drieling RL, Hersh AL. National trends in osteoporosis visits and osteoporosis treatment, 1988-2003. Arch Intern Med. 2004;164:1525–1530. doi: 10.1001/archinte.164.14.1525. [DOI] [PubMed] [Google Scholar]