Abstract
Agrin and its receptor MuSK are required for the formation of the postsynaptic apparatus at the neuromuscular junction (NMJ). In the current model the local deposition of agrin by the nerve and the resulting local activation of MuSK are responsible for creating and maintaining the postsynaptic apparatus including clusters of acetylcholine receptors (AChRs). Concomitantly, the release of acetylcholine (ACh) and the resulting depolarization disperses those postsynaptic structures that are not apposed by the nerve and thus not stabilized by agrin-MuSK signaling. Here we show that a miniaturized form of agrin, consisting of the laminin-binding and MuSK-activating domains, is sufficient to fully restore NMJs in agrin mutant mice when expressed by developing muscle. Although miniagrin is expressed uniformly throughout muscle fibers and induces ectopic AChR clusters, the size and the number of those AChR clusters contacted by the motor nerve increase during development. We provide experimental evidence that this is due to ACh, because the AChR agonist carbachol stabilizes AChR clusters in organotypic cultures of embryonic diaphragms. In summary, our results show that agrin function in NMJ development requires only two small domains, and that this function does not depend on the local deposition of agrin at synapses. Finally, they suggest a novel local function of ACh in stabilizing postsynaptic structures.
Keywords: acetylcholine, MuSK, synapse formation
One of the fundamental questions in neuroscience is how synapse formation between neurons and their targets is controlled during development. Current evidence indicates that initial stages of target recognition and synapse formation are driven by cell-adhesive interactions, and that later stages require electrical activity (1). The easy accessibility of the neuromuscular junction (NMJ) for experimental manipulation has allowed investigating synapse formation at both the molecular and physiological levels. NMJ formation critically depends on agrin, an extracellular matrix molecule released by the nerve (2, 3); the muscle-specific receptor tyrosine-kinase MuSK, which is activated by agrin (4); the low-density lipoprotein receptor-related protein 4 (5); and two intracellular adaptors, Dok-7 (6) and rapsyn (7), which bind to activated MuSK and the acetylcholine receptor (AChR), respectively. Of these, agrin and MuSK are the most upstream components, because forced expression in nonsynaptic regions of the muscle is sufficient to induce postsynaptic structures (8–11). Only certain splice variants of agrin, which differ in expression and in inserts localized in the most C-terminal laminin G (LG)-like domain, are capable of inducing AChR aggregation and MuSK activation (12).
In contrast to postsynaptic differentiation, the molecular mechanisms that initiate presynaptic differentiation are less well understood. In addition, the role of agrin at early stages of NMJ formation is still debated (13–15), because developing muscle forms AChR clusters without nerve contact (16, 17), possibly by MuSK autoactivation (18). Irrespective of whether agrin initiates (2) or merely stabilizes AChR clusters at nerve-muscle contacts, current evidence shows that agrin is important to counteract the dispersal activity of ACh (19–22). Here we report on transgenic mice that express a miniaturized version of active agrin in skeletal muscle fibers and show that this miniagrin is sufficient to restore NMJ formation in mice lacking endogenous agrin. Moreover, we provide evidence that ACh may also contribute to the selective stabilization of AChRs at nerve-muscle contacts.
Results
Transgenic Expression of a Miniaturized Form of Neural Agrin in Skeletal Muscle.
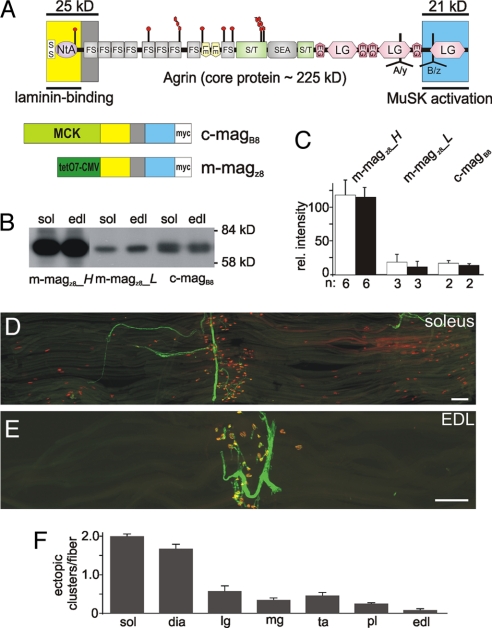
We have previously shown that a miniaturized form of neural agrin (miniagrin) containing the 8-aa insert at the B/z site is sufficient to induce postsynapse-like structures when expressed in nonsynaptic regions of the adult soleus muscle (23). To further study this phenomenon, we generated several transgenic mouse lines expressing miniagrin derived from full-length chick or mouse agrin (see Fig. 1A for domain organization). Expression of the cDNAs encoding chick miniagrin (c-magB8) or mouse miniagrin (m-magz8) was under the control of the promoter elements of muscle creatine kinase (MCK), either directly (24) or via an inducible tet-off system (see ref. 25 for further details). Several mouse lines were established, which expressed the transgenes at different levels as determined by Western blot analysis of equal amounts of muscle extracts (Fig. 1B). Equal loading was confirmed by Ponceaus S staining of the immunoblots (data not shown), and individual bands were quantified (Fig. 1C). Such quantification showed that the levels of the transgene varied between mouse lines up to 7-fold, but that there was no significant difference between soleus and extensor digitorum longus (EDL) muscle within one line. Despite the large difference in expression between the lines, we did not detect substantial differences in their biological responses. As expected for the MCK promoter (26), c-magB8 was expressed as early as embryonic day 13.5 (E13.5) [supporting information (SI) Fig. S1 A and B]. In adult muscle, c-magB8 was not enriched at NMJs (Fig. S1 C and D). Moreover, the transgene was not expressed in embryonic and adult brain or spinal cord as determined by RT-PCR (Fig. S1E). A similar expression pattern of the transgene was observed in each of the tet-off lines that expressed m-magz8 (data not shown).
Fig. 1.
Transgenic expression of a miniaturized form of neural agrin induces the formation of nonsynaptic AChR clusters. (A) (Upper) Schematic scheme of the protein domains of agrin and the localization of the alternative mRNA splice sites A/y and B/z (modified from 12). (Lower) Schematic representation of miniagrin constructs used. Promoters that drive expression in skeletal muscle are shown in green and the domain encoded by the miniagrins in the colors using in the schematic scheme (Upper). Constructs encode a myc-tag (white) for detection. (B) Western blot analysis of muscle extracts of 6-week-old mice from different transgenic lines using anti-myc antibodies. Equal amount of protein was loaded into each lane and controlled by Ponceau S staining of the blot (not shown). (C) Quantification of the signals observed in Western blots (soleus: open bars; EDL: filled bars). Bars represent mean ± SD; n indicates number of samples. (D and E) Single fiber layer bundles of soleus (D) or EDL (E) muscles isolated from 6-week-old c-magB8 transgenic mice stained for AChRs (red) and motor nerves (green). (F) Quantification of the number of ectopic AChR clusters per muscle fiber. Numbers represent mean ± SEM; n = 3 mice. In each mouse between 87 and 424 muscle fibers were examined. sol: soleus; dia: diaphragm; lg: lateral gastrocnemius; mg: medial gastrocnemius; ta: tibialis anterior; pl: plantaris; edl: extensor digitorum longus. (Scale bars: 250 μm.)
To test whether overexpression of magB/z8 induces the formation of ectopic postsynaptic structures, we examined hindlimb and diaphragm muscles in adult animals. In line with previous results (23), all of the muscles expressed ectopic AChR clusters. Using whole-mount preparations of single muscle fibers, we noticed, however, a large difference between muscles. For example, soleus muscle contained many ectopic AChR clusters (Fig. 1D), whereas EDL contained only very few (Fig. 1E). Quantification revealed that the average number of ectopic AChR clusters per muscle fiber ranged from 2 (soleus) to 0.1 (EDL) (Fig. 1F). This 20-fold difference in the response between soleus and EDL muscles was not due to differences in levels of the c-magB8 (Fig. 1 B and C). Moreover, the order of responsiveness was the same in all of the transgenic lines irrespective of the levels of magB/z8 (data not shown). Denervation of hindlimb muscles in the transgenic mice resulted in the formation of an exuberant number of AChR clusters in both muscles, and the difference in cluster number between muscles was abrogated (data not shown). These results thus indicate that muscles have different sensitivities to form ectopic AChR clusters in response to magB/z8.
Miniagrin Expressed by Skeletal Muscle Is Sufficient to Drive Synapse Formation in the Absence of Endogenous Agrin.
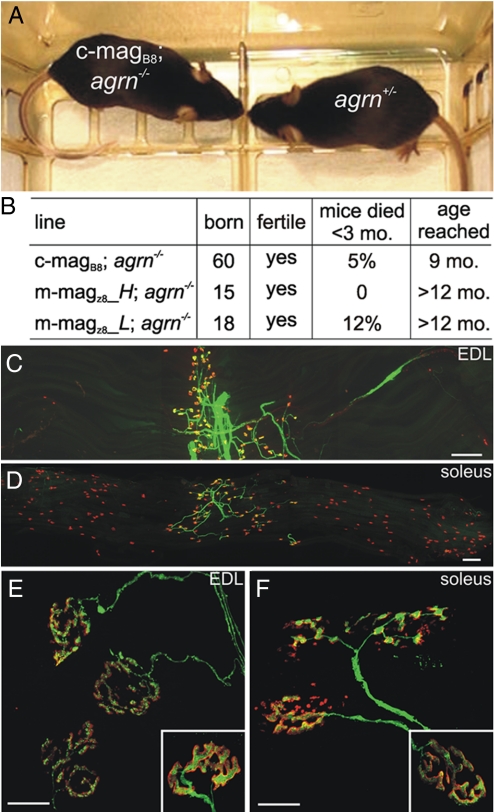
We have previously shown that expression of full-length chick agrin in motor neurons prevents the perinatal death of agrin-deficient mice (27). The relatively low number of ectopic postsynapses, the finding that the innervation band remained localized in the center of the muscle, and the lack of an overt phenotype in the magB/z8 transgenic mice led us to test whether NMJs would still form in the absence of any nerve-derived agrin. To investigate this, we mated the transgenic mice with heterozygous agrin-deficient mice (17) to obtain lines deficient for agrin and that express magB/z8 in skeletal muscle (magB/z8; agrn−/−). Recent evidence has shown that those agrin-deficient mice still synthesize a small N-terminal fragment (28), which does not affect the NMJ phenotype (17). Mice deficient for agrin and transgenic for miniagrin were born alive and could not be distinguished from their littermate controls (Fig. 2A; see also Movie S1). The majority was fertile and lived for a prolonged time, the oldest being now >1 year old. Some mice developed symptoms such as kyphosis, signs of muscle fibrillation, and eventually died early (Fig. 2B). NMJs in the magB/z8; agrn−/− mice were localized to the central region of the muscle both in EDL (Fig. 2C) and soleus (Fig. 2D). At higher magnification, the NMJs of the magB/z8; agrn−/− (Fig. 2 E and F) mice looked remarkably similar to those of control littermates (Insets in Fig. 2 E and F). In summary, these experiments show that uniform expression of miniagrin in skeletal muscle restores the formation of nerve–muscle synapses, notably also of presynaptic nerve terminals. Of interest, the rescue is superior to that obtained by the expression of full-length agrin in motor neurons (27).
Fig. 2.
Transgenic expression of magB/z8 in muscle restores NMJ function and prevents perinatal death of agrin mutant mice. (A) Photograph of two 8-week-old littermates. No difference is seen between c-magB8; agrn−/− and a control (agrn+/−) littermate. (B) Summary of the overall phenotype of rescued mice using three different transgenic mouse lines. The current age of the oldest mice is given (fifth column). See also Movie S1. (C and D) Single-fiber layer bundles of EDL (C) and soleus (D) muscle from 7-week-old m-magz8; agrn−/− mice. AChRs are visualized by Alexa-555-BTX (red), motor nerves by YFP (ref. 42; green). (Scale bar: 250 μm.) (E and F) Confocal images of NMJs of m-magz8; agrn−/− mice in the EDL (E) and soleus (F) muscle. For comparison, NMJs of control mice are shown in Insets. NMJs of rescued mice appear slightly more fragmented. (Scale bar: 50 μm.)
Development of NMJs.
To monitor the development of the NMJ and to compare our study with those of others (e.g., refs. 17 and 21), we next examined the development of the NMJ in the diaphragm from E13.5 to E18.5. To minimize biological variation, we always compared the different genotypes within the same litter. As controls, we used mice that were not transgenic and carried at least one wild-type allele for agrn (agrn+/?). These were then compared with agrin-deficient (agrn−/−), transgenic control (magB/z8; agrn+/?) and transgenic, agrin-deficient mice (magB/z8; agrn−/−). Diaphragms were isolated from embryos, and both the presynaptic nerve terminals and postsynaptic AChR clusters were visualized in whole-mount preparations (see Figs. S2–S5).
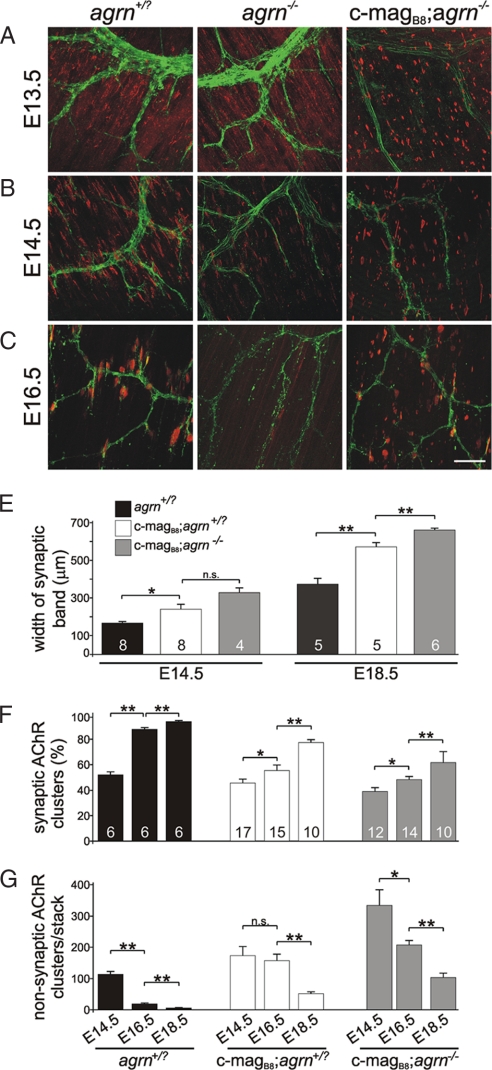
At E13.5, diaphragms from mice expressing magB/z8 contained more AChR clusters than those from control and agrin-deficient mice, but most of them were not contacted by nerve terminals (Fig. 3A; Fig. S2). At E14.5, the number, intensity and the fraction of AChR clusters associated with presynaptic nerve terminals had increased in all genotypes except in agrn−/− mice (Fig. 3B; Fig. S3). In c-magB8; agrn−/− mice, nonsynaptic AChR clusters remained more frequent than in controls (Fig. 3B). At E16.5, the majority of AChR clusters in control mice were confined to a central band, where they were contacted by nerves (Fig. 3C; Fig. S4), whereas in agrin-deficient mice, only remnants of AChR clusters were detected (Fig. 3C). Nerve association of AChR clusters was also increased in c-magB8; agrn−/− mice, although the number of nerve-free AChR clusters remained substantial (Fig. 3C; Fig. S4). At E18.5, NMJs looked more mature as the association of AChR clusters with nerve terminals became more frequent in control and magB/z8 transgenic mice (Fig. S5). In agrn−/− diaphragms, most AChR clusters had disappeared (Fig. S5). Unlike control diaphragms, however, those expressing miniagrin still contained nonsynaptic AChR clusters, and their innervation band was substantially wider than in controls (Fig. S5; see Fig. 3D for quantification).
Fig. 3.
Development of the NMJ. (A–C) Confocal images of diaphragms from E13.5 to E16.5 mice of the different genotypes indicated at the top. Pictures represent maximal intensity projections from the diaphragms shown in Figs. S2–S5. (Scale bar: 50 μm.) (D) Quantification of the width of the synaptic band. (E) In all genotypes, the relative number of synaptic (nerve-contacted) AChR clusters increases over time. (F) Conversely, the number of nonsynaptic AChR clusters per stack decreases over time. Data represent mean ± SEM of one stack of confocal images. For each parameter, diaphragms from at least three mice were examined and for each hemidiaphragm, three to eight stacks of confocal images were measured (see Fig. S3–S5 as examples). Numbers of stacks measured are given (D and E). P values (two-tailed Student's t test): **, P ≤ 0.01; *, P ≤ 0.05; n.s., P > 0.05. For experimental details, see Materials and Methods and SI Materials and Methods.
AChR Clusters Contacted by the Nerve Are Stabilized.
The current model proposes that neural agrin deposited at the site of innervation stabilizes AChR clusters, whereas ACh acts as a dispersal factor to remove nonsynaptic AChR clusters that are devoid of neural agrin (13). Because miniagrin is secreted throughout the entire length of the muscle fibers in the magB/z8 transgenic mice, all AChR clusters, irrespective of innervation, should be stable. To test this, we systematically recorded confocal stacks through the entire thickness of hemidiaphragms from E14.5 to E18.5 (see detailed description in Materials and Methods and Figs. S3–S5). When we compared the number of AChR clusters per stack, it was higher in c-magB8 transgenic mice than in controls at each time point (Fig. S6A). In control mice, the percentage of AChR clusters apposed by a nerve terminal (i.e., synaptic AChR clusters) steadily increased over time to reach close to 100% by E18.5 (Fig. 3E), whereas the number of nonsynaptic AChR clusters decreased (Fig. 3F). In c-magB8; agrn+/? mice and c-magB8; agrn−/− mice, the percentage of synaptic AChR clusters also increased (Fig. 3E), and the number of nonsynaptic clusters decreased over time (Fig. 3F). Synaptic AChR clusters were also significantly larger than those that were not innervated, and the difference became more pronounced at later developmental time points (Fig. S6B). Thus, even when nerve terminals do not secrete agrin, those AChR clusters that are contacted by nerve terminals are selectively maintained and grow in size. These results strongly suggest that nerve terminals synthesize factors in addition to agrin that stabilize AChR clusters at the synapse.
Carbachol Prevents Loss of Synaptic AChR Clusters.
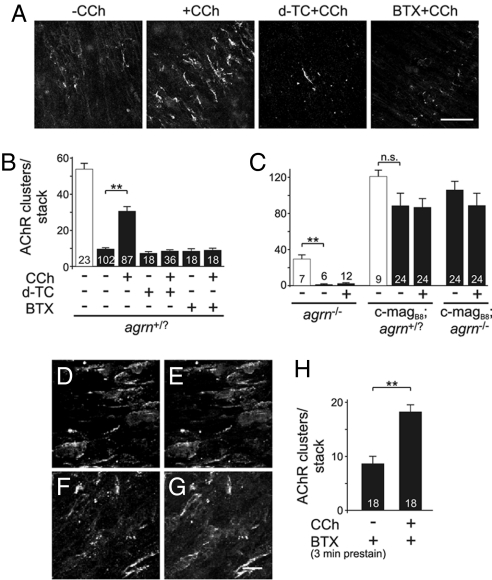
A candidate for a nerve-derived factor contributing to the stabilization of synaptic AChR clusters could be the neurotransmitter itself. To test for this, we made use of an ex vivo preparation of diaphragms isolated from E14.5 embryos. In this experimental paradigm (14), the number of AChR clusters is determined before and after culturing the excised diaphragms for 18 h at 37°C. Under control conditions, only ≈20% of the AChR clusters remained after the 18 h of incubation (Fig. 4A Left; Fig. 4B). In contrast, when diaphragms were incubated in the presence of 0.1 mM of the AChR agonist carbachol (CCh), >50% of the AChR clusters remained (Fig. 4 A and B). The effect of CCh was specific for its cholinergic action, because an excess of the AChR antagonists d-tubocurarine (d-TC) or α-bungarotoxin (BTX) abolished it (Fig. 4 A and B). The effect of CCh in preventing the loss of AChR clusters was not seen in diaphragms isolated from agrn−/− mice (Fig. 4C Left) or from magB/z8 transgenic mice (Fig. 4C Right). Thus, CCh does not prevent the loss of spontaneously formed AChR clusters, and an excess of agrin saturates the AChR aggregation response. To further test whether the effect of CCh on nerve-contacted AChR clusters was by stabilizing preexisting clusters or by triggering their new synthesis, we labeled AChRs by Alexa-488-BTX partially (Fig. 4 D and E). After the partial labeling, diaphragms were incubated for 18 h in the presence of 0.1 mM CCh. As shown in Fig. 4 F and G, AChR clusters were still labeled. As expected from a partial blockade of AChRs, the rescue of the AChR clusters by CCh was smaller, although still highly significant compared to medium alone (Fig. 4H). Although CCh had a strong effect on the number of AChR clusters, their size was not affected at all (Fig. S7). In summary, our results indicate that CCh prevents the loss of those AChR clusters that are formed during development in response to neural agrin.
Fig. 4.
Carbachol prevents the loss of AChR clusters at nerve–muscle contacts after incubation for 18 h. (A) Confocal images of AChR clusters of E14.5 diaphragms after 18-h cultivation under the conditions indicated. Under control conditions, only few AChR clusters remain (−CCh), whereas in the presence of 0.1 mM carbachol (+CCh), many AChR clusters can be detected. The effect of CCh can be abolished by an excess of d-TC or BTX. (B) Quantification of the effect of CCh in control mice. Open bar represents the number of AChR clusters before incubation, filled bars after 18-h cultivation. (C) The effect of CCh is not observed in the absence of any agrin (diaphragms isolated from agrn−/− mice) and is overwritten by constant secretion of miniagrin (diaphragms isolated from c-magB8 transgenic mice). (D–H) Effect of CCh in partially blocked diaphragm. Diaphragms from control mice were prestained with 5 μg/ml of Alexa-488-BTX for 3 min (D and F) and either fixed immediately with PFA (D and E) or incubated for 18 h in presence of 0.1 mM CCh (F and G). Diaphragms were poststained with Alexa-555-BTX (E and G). Data represent mean ± SEM from 6 independent experiments. P values (one-tailed Student's t test): **, P ≤ 0.01; *, P ≤ 0.05; n.s., not significant.
Discussion
Our work provides insights into several aspects of how NMJs form during development and, in particular, of how agrin-MuSK signaling regulates this process. The starting point of our work was the observation that mice overexpressing magB/z8 did not show an overt phenotype despite the presence of ectopic AChR clusters in the skeletal muscle. We also observed a large difference in the response of muscles to the overexpression of magB/z8. Muscles have also been shown to respond differently to innervation (29). In that work, muscles like soleus or diaphragm were categorized as “delayed synapsing” (DeSyn), whereas EDL and gastrocnemius muscles were characterized as “fast synapsing” (FaSyn). We find now that DeSyn muscles respond well to the miniagrin transgene, whereas FaSyn muscles respond poorly. One possible explanation for such a muscle-intrinsic difference to respond to innervation and to neural agrin could be a difference in the expression of molecules involved in agrin-MuSK signaling, such as MuSK itself or Dok-7 (6). Indeed, MuSK expression is substantially lower in the less-responsive EDL than in the highly responsive soleus (S.L., M.M., and M.A.R., unpublished observations).
Agrin Domains Sufficient for Postsynaptic Differentiation.
Agrin is a large heparan sulfate proteoglycan that has been shown to bind to several cell surface receptors (integrins, α-dystroglycan, and N-CAM), to growth factors (FGF), and extracellular matrix (ECM) molecules (laminins, other heparan sulfate proteoglycans; for review, see ref. 12). That the miniaturized form of neural agrin consisting solely of the laminin-binding (30) and the MuSK-activating domain (31, 32) can rescue the perinatal death caused by agrin deficiency strongly argues that none of the other interactions of full-length agrin are required for its function in the initial development of NMJs. Of particular interest is that our work provides in vivo evidence that induction of postsynaptic structures during development does not require binding of neural agrin to α-dystroglycan, unlike what was postulated previously (33).
Our data also show that presynaptic differentiation is not a direct consequence of the accumulation of agrin at nerve–muscle contacts as had been postulated (34, 35). Instead, presynapses are rather formed as a consequence of the accumulation of other factor(s) that become concentrated in response to agrin-MuSK signaling during postsynaptic differentiation. Consistent with this idea, motor nerves continue to grow in MuSK-deficient mice (4), and overexpression of MuSK in skeletal muscle, which causes its self-activation and the formation of postsynapses, is sufficient to induce presynaptic differentiation in agrn−/− mice (18). One possibility is that activation of MuSK could also induce presynaptic specialization. Such a direct feedback of activated MuSK to motor neurons has been described in vitro (36). Alternatively, factors may accumulate during postsynaptic differentiation and they, in turn, may induce presynaptic differentiation. Proteins implicated in presynaptic differentiation are FGFs, laminin-β2, and collagen α(IV) chains (37).
The Central Region of the Muscle Is More Responsive to Miniagrin During Development.
Our work also provides evidence that the uniform expression of miniagrin throughout the muscle influences the pattern of innervation, because we observed a substantial widening of the synaptic band in magB/z8 mice (Fig. 3D). There are several reasons that may underlie this effect. For example, high concentrations of magB/z8 will activate MuSK in more lateral regions of the muscle where the levels of MuSK are low (18). This activation would then trigger transcriptional changes that allow recruitment of more MuSK (10) and thus widen the region in which NMJs form. Because a widening of the innervation band was also observed in mice overexpressing MuSK (18), our results indicate that hyperactivation of MuSK by adding neural agrin or by overexpression of MuSK itself can equally well cause a widening of the innervation band (see also ref. 14 for discussion).
The AChR Agonist Carbachol Stabilizes AChR Clusters.
We show here that the relative number of nerve-contacted AChR clusters increases (Fig. 3E), and that the number of nonsynaptic AChR clusters decreases during development (Fig. 3F), despite the uniform expression of miniagrin. Moreover, we show that the size of nerve-contacted AChR clusters is significantly larger than that of nonsynaptic ones (Fig. S6B). Thus, nerve-contacted postsynapses are preferentially maintained in magB/z8; agrn−/− animals, whereas those not innervated are eliminated, indicating that motor nerve terminals produce factors in addition to agrin that stabilize synaptic AChR clusters. Our data now indicate that one such factor might be ACh itself, because addition of the AChR agonist CCh prevents the loss of AChR clusters in cultured, denervated diaphragm muscle (Fig. 4 A and B). Our results also show that the effect of CCh is based on its cholinergic function as it is blocked by an excess of the AChR antagonists d-TC or BTX (Fig. 4 A and B) and that CCh exerts its effect only together with neural agrin (Fig. 4C). An additional support for the conclusion that the AChR clusters maintained by CCh in this ex vivo preparation are those that were initially innervated by motor neurons is that they are localized in the middle of the diaphragm, reminiscent to the central band of innervation (Fig. 4A). Finally, we also show that at least part of the effect of CCh is based on its stabilizing preexisting AChR clusters (Fig. 4 D–H).
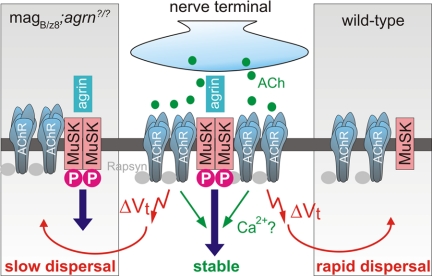
In current models, the only role of ACh during NMJ formation is that of a dispersal factor for the spontaneously formed AChR clusters, whereas the local deposition of agrin in synaptic basal lamina and thus activation of MuSK counteracts this dispersal activity of ACh (Fig. 5Right). This model is based on the observation that spontaneously formed AChR clusters that are not innervated disperse in wild-type mice whereas they remain in mice deficient for choline acetyltransferase (ChAT; refs. 19, 20). Moreover, in agrin-deficient mice, nerve-associated AChR clusters are lost during development, whereas they are maintained in mice deficient for both agrin and ChAT (21). Finally, addition of CCh to cultured myotubes disperses spontaneously formed AChR clusters, whereas AChR clusters become resistant to this dispersal if they are induced by agrin (21, 22). Based on our experiments, we now propose a modified model in which local signaling pathways activated by the opening of AChRs also contribute to the stabilization of synaptic AChR clusters (Fig. 5). According to our model, aneural postsynaptic structures in wild-type mice will disperse rapidly, because they lack both the local AChR-mediated and the agrin-MuSK signal. Nonsynaptic AChR clusters in magB/z8 transgenic mice still disperse, although more slowly than in wild-type mice, because they are not stabilized by the local action of ACh (Fig. 5). Such a proposed local action of ACh on the stability of postsynaptic AChRs during development is consistent with the observation that blockade of ACh release decreases the half-life of synaptic AChRs from 14 days to 2 h at mature mouse NMJs (38).
Fig. 5.
Proposed model for the action of acetylcholine to stabilize AChR clusters. At the NMJ, release of agrin causes MuSK dimerization and phosphorylation. This activates intracellular signaling pathways. Release of ACh from the presynaptic nerve terminal depolarizes the muscle fiber (ΔVt) and disperses aneural AChR clusters (red arrow). ACh also causes the opening of AChRs and stabilizes them at sites of agrin-MuSK signaling. Dispersal of aneural AChR clusters in wild-type mice, which are not stabilized by agrin-MuSK signaling is fast (right part of the scheme). In magB/z8 transgenic mice, aneural AChR clusters are stabilized by agrin-MuSK signaling (left part of the scheme) but most of them still disperse slowly as they lack the local ACh signal from the nerve terminal. One possibility for the stabilizing function of ACh on postsynaptic structures is the local influx of Ca2+ via AChRs that could act in parallel with agrin-MuSK signaling.
What could be the intracellular signal that triggers the maintenance of AChRs? One possibility is that the opening of AChRs by ACh causes the local influx of calcium (39). Because calcium has been shown to be required for agrin-induced AChR clustering and cluster maintenance in vitro (40), the high calcium concentration in conjunction with activation of agrin-MuSK signaling could result in sustained stabilization of postsynaptic structures (Fig. 5C). Interestingly, an influence of calcium influx via AChR channels on innervation has been suggested previously, because changing the dynamics of AChR-mediated calcium influx by genetically engineering fetal-type AChRs to adopt the ion conductance properties of adult-type AChRs causes a widening of the synaptic band (39, 41). In summary, our results suggest a role of ACh in the stabilization of postsynaptic receptors. A dual role of neurotransmitters to locally stabilize the postsynaptic receptor clusters that are apposed by a nerve terminal and to destabilize those that are not innervated by the appropriate presynaptic nerve terminal may also be involved in synapse formation and selective synapse elimination in the CNS.
Materials and Methods
Details on the different mouse models, antibodies used, immunoblots, isolation, and staining of diaphragms and the quantitative analysis of synaptic AChR cluster bands are given in SI Materials and Methods.
Organotypic Culture of E14.5 Diaphragms.
The method of culturing E14.5 diaphragms ex vivo has been described (14). Briefly, diaphragms with ribcages were incubated in M199 medium supplemented with 5% horse serum and penicillin/streptomycin in an atmosphere of 95% O2 and 5% CO2 at 37°C. Carbachol (Sigma) was used at a concentration of 0.1 mM. In blocking experiments, medium was supplemented with 1 mM d-tubocurarine or 2 μg/ml of Alexa-488-BTX. Preexisting AChRs were labeled for 3 min with 5 μg/ml of Alexa-488-BTX. After 18-h culture, diaphragms were fixed in 4% PFA, stained with Alexa-555-BTX, and mounted. In the middle of each hemidiaphragm, three confocal stacks were recorded with the ×40 objective and 0.5-μm z-steps. The number and volume of AChR clusters (>10 μm3) were quantified as described (14).
Image Acquisition and Processing.
Diaphragms were analyzed with a confocal laser scanning microscope (Leica TCS SPE). Images were recorded with ×20 or ×40 objectives with 1- and 0.5-μm z-steps, respectively. The same laser power and parameter setting were applied. For quantification of AChR clusters, three to eight image stacks with a ×20 objective in the middle of the hemidiaphragm (as illustrated in Fig. S3–S5) were analyzed with Imaris software (Bitplane). The threshold intensity was set by visual inspection, so that the size of the AChR clusters and the nerve was similar to what was shown in the image stack. Only particles that were >30 μm3 were included. The total number and the volume of AChR clusters were determined as described (14).
Supplementary Material
Acknowledgments.
We thank F. Schnüriger and G. Bittcher for support in the initial stages of the project. We are grateful to J. L. Bixby for comments on the manuscript and to L. Landmann for help with confocal microscopy and quantification. The Transgenic Mouse Core Facility of the University of Basel is acknowledged for generating the transgenic mice, Dr. J. R. Sanes for providing the agrin knockout mice, and Dr. N. Raben for the MCK-tTA mice. This work was supported by grants from the Swiss National Science Foundation, the Swiss Foundation for Research on Muscle Diseases, and the Cantons of Basel-Stadt and Baselland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801683105/DCSupplemental.
References
- 1.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahan UJ. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Gautam M, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 4.DeChiara TM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 5.Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 6.Okada K, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- 7.Gautam M, et al. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- 8.Jones G, et al. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc Natl Acad Sci USA. 1997;94:2654–2659. doi: 10.1073/pnas.94.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen I, Rimer M, Lomo T, McMahan UJ. Agrin-induced postsynaptic apparatus in skeletal muscle fibers in vivo. Mol Cell Neurosci. 1997;9:237–253. doi: 10.1006/mcne.1997.0623. [DOI] [PubMed] [Google Scholar]
- 10.Jones G, Moore C, Hashemolhosseini S, Brenner HR. Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J Neurosci. 1999;19:3376–3383. doi: 10.1523/JNEUROSCI.19-09-03376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sander A, Hesser BA, Witzemann V. MuSK induces in vivo acetylcholine receptor clusters in a ligand-independent manner. J Cell Biol. 2001;155:1287–1296. doi: 10.1083/jcb.200105034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezakova G, Ruegg MA. New insights into the roles of agrin. Nat Rev Mol Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- 13.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Landmann L, Ruegg MA, Brenner HR. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J Neurosci. 2008;28:3333–3340. doi: 10.1523/JNEUROSCI.5590-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vock VM, Ponomareva ON, Rimer M. Evidence for muscle-dependent neuromuscular synaptic site determination in mammals. J Neurosci. 2008;28:3123–3130. doi: 10.1523/JNEUROSCI.5080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIbeta and neural development. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]
- 17.Lin W, et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 18.Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nat Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misgeld T, et al. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 20.Brandon EP, et al. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J Neurosci. 2003;23:539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Meier T, et al. A minigene of neural agrin encoding the laminin-binding and acetylcholine receptor-aggregating domains is sufficient to induce postsynaptic differentiation in muscle fibres. Eur J Neurosci. 1998;10:3141–3152. doi: 10.1046/j.1460-9568.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- 24.Moll J, et al. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 25.Meinen S, Barzaghi P, Lin S, Lochmuller H, Ruegg MA. Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages. J Cell Biol. 2007;176:979–993. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sternberg EA, et al. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol Cell Biol. 1988;8:2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ksiazek I, et al. Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci. 2007;27:7183–7195. doi: 10.1523/JNEUROSCI.1609-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey SJ, et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171:139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pun S, et al. An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron. 2002;34:357–370. doi: 10.1016/s0896-6273(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 30.Denzer AJ, Brandenberger R, Gesemann M, Chiquet M, Ruegg MA. Agrin binds to the nerve-muscle basal lamina via laminin. J Cell Biol. 1997;137:671–683. doi: 10.1083/jcb.137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotton P, et al. Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. J Biol Chem. 2006;281:36835–36845. doi: 10.1074/jbc.M607887200. [DOI] [PubMed] [Google Scholar]
- 33.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 34.Campagna JA, Ruegg MA, Bixby JL. Agrin is a differentiation-inducing “stop signal” for motoneurons in vitro. Neuron. 1995;15:1365–1374. doi: 10.1016/0896-6273(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 35.Chang D, Woo JS, Campanelli J, Scheller RH, Ignatius MJ. Agrin inhibits neurite outgrowth but promotes attachment of embryonic motor and sensory neurons. Dev Biol. 1997;181:21–35. doi: 10.1006/dbio.1996.8435. [DOI] [PubMed] [Google Scholar]
- 36.Dimitropoulou A, Bixby JL. Motor neurite outgrowth is selectively inhibited by cell surface MuSK and agrin. Mol Cell Neurosci. 2005;28:292–302. doi: 10.1016/j.mcn.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Fox MA, et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 38.Akaaboune M, Culican SM, Turney SG, Lichtman JW. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. doi: 10.1126/science.286.5439.503. [DOI] [PubMed] [Google Scholar]
- 39.Villarroel A, Sakmann B. Calcium permeability increase of endplate channels in rat muscle during postnatal development. J Physiol. 1996;496:331–338. doi: 10.1113/jphysiol.1996.sp021688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Megeath LJ, Fallon JR. Intracellular calcium regulates agrin-induced acetylcholine receptor clustering. J Neurosci. 1998;18:672–678. doi: 10.1523/JNEUROSCI.18-02-00672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenen M, Peter C, Villarroel A, Witzemann V, Sakmann B. Acetylcholine receptor channel subtype directs the innervation pattern of skeletal muscle. EMBO Rep. 2005;6:570–576. doi: 10.1038/sj.embor.7400429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.