Abstract
Here we report a method for performing a chronic constriction injury (CCI) of the infraorbital nerve (ION) in the rat as a component of a chronic pain model. The surgical approach to the ION is described together with the use of a modified dental syringe needle that simplifies placing two chromic gut ligatures around the ION. This method makes the surgical procedure easier, the nerve injury more consistent across animals and reduces secondary damage to the ION and surrounding tissue. Pain behavior testing together with immunostaining for markers of nerve injury in the spinal trigeminal nucleus show the suitability of this procedure as a model of orofacial pain.
Keywords: CCI, ION, trigeminal ganglion, technique, neuropathy, chronic pain, animal model, microsurgery
1. Introduction
Since Bennett et al (Bennett and Xie, 1988) developed the chronic constriction injury (CCI) many laboratories have used this procedure to study different aspects of chronic neuropathic pain (Benoist et al., 1999; Imamura et al., 1997; Kitagawa et al., 2006; Vos and Strassman, 1995; Vos et al., 1994; Waite and de Permentier, 1991). CCI involves tying one or more loose chromic gut ligatures around a peripheral nerve, resulting in lowered threshold to nociceptive as well as innocuous stimuli that develops within 2 to 3 days after surgery and lasts for at least a month (Bennett and Xie, 1988). Chromic gut rather than silk suture is used because the chemical toxicity of the gut is thought to enhance the development of the pain behaviors observed following CCI (Maves et al., 1993).
Most commonly, the CCI is applied to the sciatic nerve, which is a relatively straightforward surgical target, and results in changed sensory thresholds in the affected rear limb. CCI of branches of the trigeminal nerve has also been used to study idiopathic facial pain such as tic douloureux or secondary trigeminal neuralgia, pathologies that are notoriously difficult to treat (Fisher et al., 2003). An advantage of using the trigeminal nerve for pain related studies is that the nerve carries no autonomic component and the motor component is confined to the mandibular division of the nerve. Thus using a branch of the trigeminal other than the mandibular makes interpretation of sensory changes following CCI easier to interpret. Usually a single branch of the trigeminal nerve is ligatured and the branch most commonly selected is the infraorbital nerve (ION) that forms almost the entire second (V2, maxillary) division of the trigeminal nerve although other branches such as the mental nerve (Grelik et al., 2005) have been ligatured. The ION innervates the snout including the vibrissal pad, the upper teeth, part of the rhinarium and dorsal part of the oral cavity (Waite and de Permentier, 1991).
One complication in using the trigeminal nerve for a CCI is that the surgical approach can be more complex than for other peripheral nerves such as the sciatic. The earliest description of accessing the ION required the removal of the eye (Gregg, 1973). Modifications of this method also removed the eye but were used for cutting the nerve (Jacquin and Zeigler, 1983) rather than placing ligatures. More recently, a retro orbital approach has been used for placing ligatures (Vos et al., 1994) but there is no detailed description of the procedure and the surgical approach is not as straightforward as CCI of the sciatic nerve. Here we describe a method for placing ligatures around the ION that simplifies the procedure and minimizes unwanted secondary damage to the nerve and surrounding tissue.
2. Materials and methods
2.1. Ligature placement aided by modified dental needle
The principal problem with placing the ligatures around the ION is the location of the nerve deep within the orbit necessitating temporary retraction of the overlying orbit contents to gain access to the nerve. We have found that using a modified dental needle aids placing the ligatures with minimal disturbance of the eye. The needles should be prepared before surgery.
2.1.1. Inserting the chromic gut into the needle
Eight centimeters of 5-0 chromic gut suture (www.ethicon.com) is threaded into the back end of a 25 gauge, 1 ¾ inch dental needle (www2.henryschein.com, Monoject 25 gauge long, red). A dental needle is used because the back end of the needle extends 5mm past the hub (Fig 1A) which simplifies threading the chromic gut. Threading the chromic gut into the needle is aided by cutting the chromic gut at a sharp angle and allowing the gut to dry a little so that it becomes stiffer and easier to thread.
Figure 1.
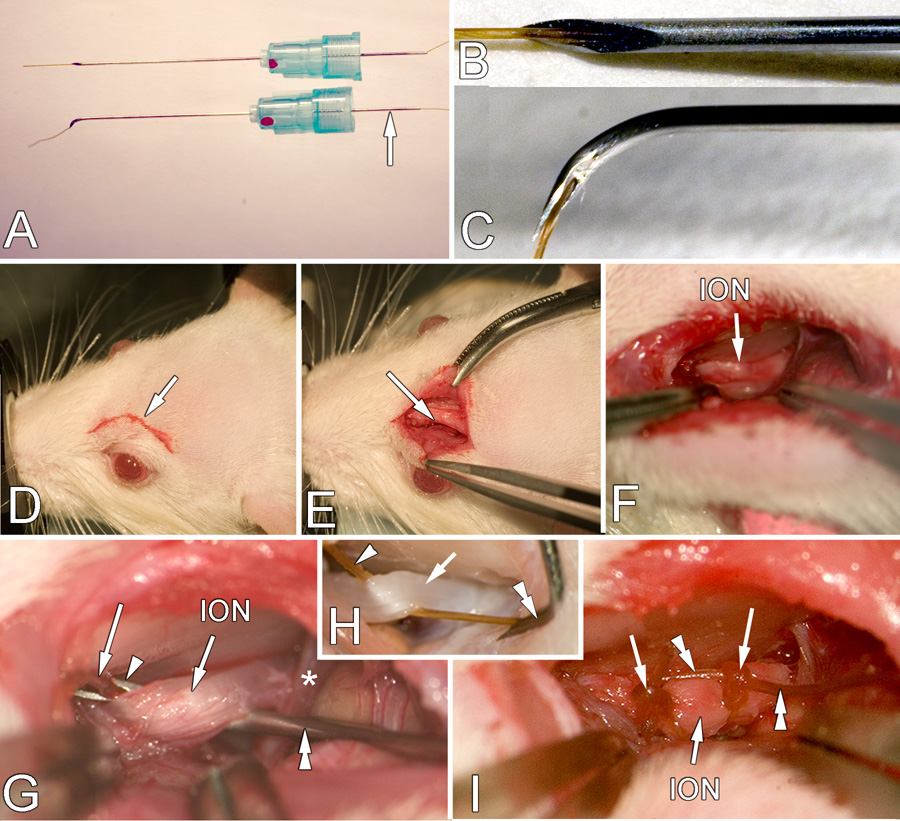
A. A dental needle threaded with chromic gut suture (top neele) and with tip bent (bottom needle). The arrow indicates the needle extension found on dental needles that simplifies threading. B, C. High magnification of the threaded tip before and after bending, respectively. D. Location and size of the initial incision (arrow) E. Retraction of the skin to expose the edge of the frontal bone (arrow). F. Retraction of the orbital contents to show deep location of the ION (arrow). G. High magnification of the placement of the ligature. The syringe needle (double arrowheads) has been slid under the ION and the needle orifice (arrowhead) and protruding gut suture (arrow) is visible. The component of the opthalmic nerve (*) is also seen. H. This image is from a perfused rat with the orbit contents removed to more clearly show the end of the gut (arrowhead) that is held by the forceps (not seen) and the direction in which the needle (double arrowhead) is withdrawn to leave the gut suture in place. I. The ION with two ligatures (arrows) in place. The double arrowheads indicate the ends of the ligature remaining after tying which will be cut off before the skin is sutured closed. NOTE: In G and H the nerve is pulled up more than normal to show the procedure more clearly.
2.1.2 Bending the needle
When 5mm of chromic gut protrudes from the needle tip (Fig. 1B), the needle tip is gently bent through 90 degrees using a small hemostat. The bending should be accomplished by several small bends to produce a smooth curve starting about 3mm from the tip rather than a single bend that results in a sharp right angle. The opening of the needle should be on the inside of the bend (Fig. 1C). A bend longer than 3 mm makes it harder to insert the needle within the limited exposure afforded by the orbit. An angle more acute than 90 degrees also makes inserting the needle between the nerve and the eye more difficult thereby increasing the likelihood of a non-ligature-related damage of the nerve. A more obtuse angle requires additional retraction of the eyes and risks damage to the ethmoidal peduncle and orbit. The threaded needle is placed in sterile saline to maintain suppleness of the suture material while bending the needle and in positioning it around the nerve.
We recommend using a new needle for each ligature, as the needles are inexpensive. However, the same needle can be re-used by threading new gut into the bent needle after sterilizing the needle. If re-using the needle it may be necessary to clean out the bore with a 0.00815 gauge Hamilton cleaning wire (www.hamiltoncompany.com). If there is concern about the nerve coming into contact with ferrous materials, the needles coated in the same manners as electrodes are coated (Verhagen et al., 2003) using commercial epoxylite (6001M. FHC Inc. Bowdoinham, ME) or thinned commercial acrylic paint.
2.2. Animals
Twenty-eight adult male Sprague–Dawley rats (220–260 g, Charles River) were used in this study. All animals were exposed to light 12 h per day; food and water were available ad libitum. Procedures for the maintenance and use of the experimental animals were approved by the Animal Care and Use Advisory Committees at UCSF and were carried out in accordance with NIH regulations on animal use.
2.3. Surgery
2.3.1. Anesthesia and head immobilization
Rats were anesthetized with a combination of Ketamine and Xylazine (90mg/kg + 5mg/kg) and supplemented if necessary with an inhalant anesthetic (1–2% Halothane 40% Oxygen and room air) delivered through a face mask with a scavenging system to maintain a level of anesthesia at which the animal does not withdraw from pinch and has no corneal blink reflex. The skin above the eye is shaved (Fig 1D) and the rat is placed in a stereotaxic frame. Ophthalmic cream is applied to the cornea of both eyes to prevent drying damage. If a stereotaxic frame is not available, the procedure can be accomplished using any arrangement that immobilizes the head.
2.3.2. Exposure of the ION
An anterior-posterior skin incision approximately 7mm long is made 2mm above the left eye, following the curve of the frontal bone (Fig 1E). The fascia and muscle is then gently teased laterally from the bone using a periosteal elevator or a scalpel blade until the contents of the orbit can be gently retracted laterally (Fig. 1E). Once the eye is retracted the ION can be seen approximately 8mm deep within the orbit, lying on the maxillary bone (Fig. 1F). Once visible, at least 5mm of the ION must be gently freed from the surrounding connective tissue with fine jeweler’s forceps using the spreading technique (Fig. 1F) in order to place the 2 ligatures. Care is taken during exposure of the ION not to damage the anterior ethmoidal nerve (Fig. 1G), a component of the ophthalmic nerve (V1). This small nerve crosses perpendicularly above the ION and may be slightly stretched when retracting the contents of the orbit.
2.3.3. Ligature placement
To place the ligature, the suture-loaded needle is inserted into the orbit cavity by positioning the needle lateral to the nerve with the bent tip parallel to the nerve (Figs. 1G, H). The needle is then turned 90 degrees to slide the tip under the nerve from lateral to medial (Fig. 1G). One advantage of this approach is that the bone acts as a natural barrier and indicates when the needle has been inserted far enough. Gentle retraction of the ION will reveal the tip of the needle and the protruding end of the gut (Fig. 1G). This step should be carried out with minimal delay to avoid blood entering the tip of the needle and coagulating and to minimize the chromic gut drying and becoming brittle. Forceps are used to grip the protruding gut and pull about 5 cm through the needle medial to the ION. The forceps are then repositioned to grasp the gut near the needle and the needle is then gently withdrawn laterally leaving the other end of the gut freely accessible on the lateral side of the ION (Fig. 1H). If the gut dries at the free, hub end, during the procedure, it can be difficult to remove the needle. Keeping all parts of the gut moist with sterile saline aids removal and once the needle has been withdrawn the gut can be moved to the desired position along the ION and a loose ligature tied (Fig. 1I). Again, keeping the free ends of the gut moist during tying will aid the procedure.
Two ligatures are placed 3–4 mm apart using the same procedure. Using the Bennett(Bennett and Xie, 1988) model we tighten the ligature until the ION is barely constricted when viewed at 40x magnification then a second knot is tied to prevent slippage. The excess gut is cut from the ligatures (Fig. 1I). The incision above the eye is sutured with 5-0 silk and the rat allowed to recover.
2.4. Behavioral testing
To show that this method of performing a CCI produces behavioral changes we present the results of von Frey hair testing. Other testing methods are available (Vit et al., 2008). For sensory testing of the skin supplied by the ION we used a procedure modified from that of Vos et al. (Vos et al., 1994). In brief, a single rat is placed in a standard size home cage and the tester reaches into the cage and stimulates the rat snout with several different weight von Frey hairs. This is done for several sessions before experimental testing begins to acclimate the rat to the environment and the presence of the tester’s hand and von Frey wand. All testers were blinded to the whether the rat had CCI or sham surgery. After the acclimatization sessions the tester was able to apply the von Frey hair to the territory of the ION (whisker pad) and record the response. Three applications are made on each side of the snout and the mean of the measurements is used. If the rat did not immediately react to the hair, the hair was left in place for 3 seconds. In this experiment 2 different von Frey filaments were used (0.4 and 2 g), one after the other, each for 3 applications with the smallest hair used first. The response to each of the filaments is scored as 0, no response; score 1, detection - the rat turns its head toward the stimulating object and the stimulus object is then explored; score 2, withdrawal reaction - the rat turns its head slowly away or moves it briskly backward when the stimulation is applied, and sometimes a single face wipe ipsilateral to the stimulated area occurs; score 3, escape/attack - the rat avoids further contact with the stimulus object, either passively by moving its body away from the stimulating object to assume a crouching position against the cage wall, or actively by attacking the stimulus object, making biting and grabbing movements; score 4, asymmetric face grooming - the rat displays an uninterrupted series of at least three face-wash strokes directed toward the stimulated facial area.
2.5 Histological analysis
All rats were deeply anesthetized with sodium pentobarbital (75 mg/kg) and perfused through the ascending aorta with an aldehyde fixative. The trigeminal ganglia, ION and brainstem were harvested. Immunohistochemistry was done according to standard protocols using primary antisera directed against ATF3 (C-19, Santa Cruz Cat #sc-188, www.scbt.com, 1:800) and OX-42 (CD3/CD11b, Pharmingen cat#22081D, www.bdbiosciences.com, 1:2000).
2.6. Data analysis
Data are expressed as the mean ±standard error of the mean (S.E.M.). Differences between treatment groups were evaluated against sham operated rats and baseline values using the Mann–Whitney U-test (two tailed) with a correction for repeated measures. P values of less than 0.05 were taken as significant.
3. Results
Rats that underwent CCI of the ION by the described procedure were used in studies of facial pain and the results will be published elsewhere. For illustrative purposes we present here an example of the behavioral and anatomical data used to document changes following the described procedure.
3.1 Behavior – von Frey testing
Following CCI of the ION there was an initial reduction in the response to mechanical stimulation in the territory of the ligatured ION (Fig. 2). This reduction is not generally reported as most studies begin testing at day 3 post-surgery (Imamura et al., 1997; Kitagawa et al., 2006; Lim et al., 2007) and could be due to residual effects of the anesthetic and analgesics given during surgery or an increase in post-surgical endogenous opioids. Beginning three days after surgery the response score increased and reached a plateau by post-surgery day 5. An increased score indicates that the rat finds the stimulation unpleasant or nociceptive. On sham operated rats the behavioral score remained at pre-surgery levels for the entire period.
Figure 2.
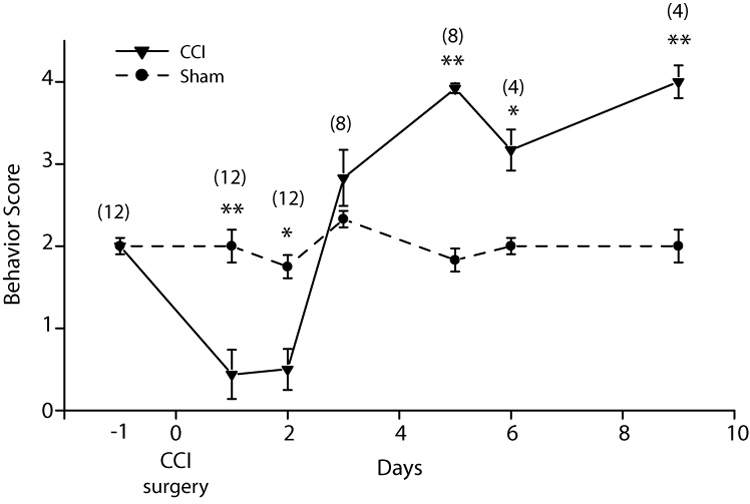
Response to von Frey hair stimulation. CCI surgery was performed on day 0. A higher behavior score indicated an increased aversive response to the von Frey hair stimulation. Following the CCI there is an initial reduction in the behavioral score followed by an increase after 2 days to reach a plateau by day 5 post-CCI The behavioral score for the sham operated animals remains unchanged. * = <0.05; ** = >0.01. The numbers in parentheses indicated the number of rats tested for CCI and sham at each time point.
3.2. Immunohistochemistry
We examined the trigeminal ganglia 5 days after CCI of the ION using antibodies for ATF3, a marker of cell injury (Obata et al., 2003). Numerous ATF3 immunopositive neurons were present in the territory of the projection of the ION (Fig. 3A). Next we examined the trigeminal nuclear complex for changes in microglial staining which is characteristic of peripheral nerve damage (Tawfik et al., 2007). There was an increase of microglia immunostaining (Ox-42) in all the trigeminal sub-nuclei, more especially in sub nucleus caudalis (Fig. 3B). The increase began 3 days after CCI of the ION. Quantitation of ATF3 immunopositive neurons from sections at 3 different dorso-ventral levels through the ganglion and counted using unbiased stereology using a computer aided stereology system (StereoInvestigator, Microbrighfield Inc. Williston, VT). Densitometry for OX-42 immunostaining was performed on digital images with the threshold set to a uniform value. The number of black pixels was counted using Scion Image software Scion Corp. Frederick, MD) in a standardized square and analyzed on three sections for each trigeminal brainstem subnucleus (caudalis, interpolaris/oralis and main sensory) per animal. The densitometry data is presented as the mean number of pixels per square micron. The densitometry was not used to measure the absolute value of immunostained marker but to determine relative differences between operated and unoperated sides.
Figure 3.
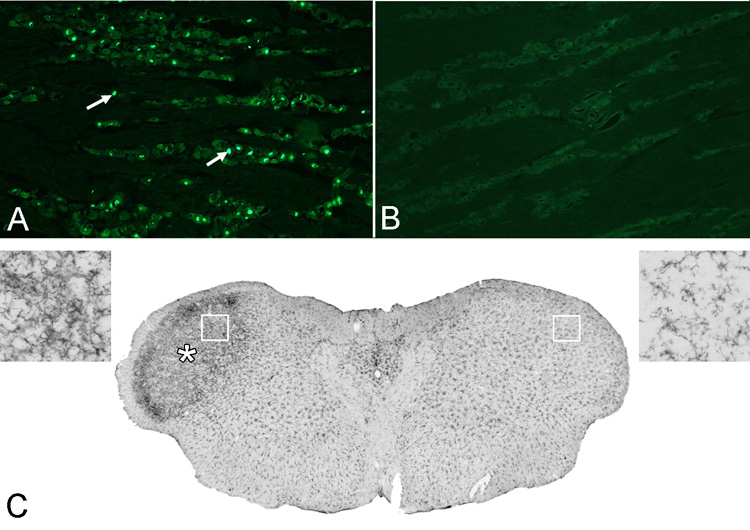
Section from the trigeminal ganglion ipsilateral (A) and contralateral (B) to the CCI immunostained for the cell injury marker ATF3. CCI produces a significant increase in ATF3 immunopositive cells (e.g. arrows) in the ganglion on the injured side (labeled neurons/4×106 µm2; ipsi, = 144.16; contra, 2.58; p<0.001, n=4). A coronal section (C) of the caudal brainstem at the level of nucleus caudalis (5.0 mm posterior to interaural 0). Five days after CCI there is a significant increase in OX-42 immunostaining (*) on the side ipsilateral to the CCI (left. Pixel intensity, ratio ipsi/contra: 3.14; P < 0.001, n=4). The insets at top left and top right show higher magnifications of the areas indicated by the boxes.
4. Discussion
4.1 Comparison with other surgical approaches
We choose the supra-orbital approach to the ION but other surgical approaches are possible. Imamura and colleagues (Imamura et al., 1997) described an intra-oral approach using a small incision made in the gingivo-buccal margin proximal to the first molar which has the advantage that it avoids any possibility of damaging skin in an area that might be used for sensory testing. However, we find the intraoral approach to be more difficult and the region of the ION that is exposed is more distal. The more distal location means that more branches have left the nerve and so a smaller portion of the nerve is included in the ligature.
Another approach is to expose the nerve by an incision in the skin of the vibrissal pad a few mm from where the ION exits the optic foramen (Henry et al., 2007). This method is the most straightforward and easiest surgically but cutting the skin of the vibrissal pad complicates sensory testing in that region. As with the intra-oral approach the ligatures are tied more distally than with the supra-orbital approach so less of the V2 division is included. The supra-orbital approach we describe places ligatures on the ION proximal to all branches. Thus the ligatures affect the largest territory. The incision is above the eye in the territory of V1 and so avoids injuring the territory covered by the ION.
4.2. Notes on supra-orbital surgery
The two most disconcerting aspects of the surgery is the retraction of the eyeball and the depth at which the nerve lies. Once one is familiar with the depth at which the nerve lies it is easier to minimize how far the eye socket contents are retracted. Careful surgery will minimize bleeding into the orbit which is important in order to obtain a clear view of the nerve for ligature placement. Packing with gel-foam or small triangular, sterile saline dampened wedges of filter paper will help control bleeding. The described procedure does not damage any of the extra-ocular muscles of the eye and following surgery the eye appears normal and there are no apparent deficits in vision.
4.3. Conclusion
We find the described method for chronic constriction injury of the infra-orbital nerve has several advantages over other approaches. Although there is a learning curve, the surgery is not complex and the use of the modified needle simplifies the procedure and minimizes non-ligature related trauma. The method results in consistent, reproducible, placement of the ligatures minimizing variation in the experimental results.
Acknowledgements
We would like to thank Ms Criselda Cua and Ms Clare Carlson for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Benoist JM, Gautron M, Guilbaud G. Experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve: changes in neuronal activities in the somatosensory cortices corresponding to the infraorbital nerve. Exp Brain Res. 1999;126:383–398. doi: 10.1007/s002210050745. [DOI] [PubMed] [Google Scholar]
- Fisher A, Zakrzewska JM, Patsalos PN. Trigeminal neuralgia: current treatments and future developments. Expert Opin Emerg Drugs. 2003;8:123–143. doi: 10.1517/14728214.8.1.123. [DOI] [PubMed] [Google Scholar]
- Gregg JM. A surgical approach to the ophthalmic-maxillary nerve trunks in the rat. Journal of dental research. 1973;52:392. doi: 10.1177/00220345730520024001. [DOI] [PubMed] [Google Scholar]
- Grelik C, Bennett GJ, Ribeiro-da-Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. The European journal of neuroscience. 2005;21:2475–2487. doi: 10.1111/j.1460-9568.2005.04089.x. [DOI] [PubMed] [Google Scholar]
- Henry MA, Freking AR, Johnson LR, Levinson SR. Sodium channel Nav1.6 accumulates at the site of infraorbital nerve injury. BMC Neurosci. 2007;8:56. doi: 10.1186/1471-2202-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Zeigler HP. Trigeminal orosensation and ingestive behavior in the rat. Behavioral neuroscience. 1983;97:62–97. doi: 10.1037//0735-7044.97.1.62. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Takeda M, Suzuki I, Kadoi J, Tsuboi Y, Honda K, Matsumoto S, Nakagawa H, Tanabe A, Iwata K. Mechanisms involved in modulation of trigeminal primary afferent activity in rats with peripheral mononeuropathy. The European journal of neuroscience. 2006;24:1976–1986. doi: 10.1111/j.1460-9568.2006.05065.x. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Jeon HJ, Yang GY, Lee MK, Ju JS, Han SR, Ahn DK. Intracisternal administration of mitogen-activated protein kinase inhibitors reduced mechanical allodynia following chronic constriction injury of infraorbital nerve in rats. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:1322–1329. doi: 10.1016/j.pnpbp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain. 1993;54:57–69. doi: 10.1016/0304-3959(93)90100-4. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain, behavior, and immunity. 2007;21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Gabbott PL, Rolls ET. A simple method for reconditioning epoxy-coated microelectrodes for extracellular single neuron recording. Journal of neuroscience methods. 2003;123:215–217. doi: 10.1016/s0165-0270(02)00365-5. [DOI] [PubMed] [Google Scholar]
- Vit J-P, Ohara PT, Bhargava A, Kelley K, Jasmin J. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. Journal of Neuroscience. 2008 doi: 10.1523/JNEUROSCI.5053-07.2008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BP, Strassman AM. Fos expression in the medullary dorsal horn of the rat after chronic constriction injury to the infraorbital nerve. J Comp Neurol. 1995;357:362–375. doi: 10.1002/cne.903570304. [DOI] [PubMed] [Google Scholar]
- Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite PM, de Permentier P. The rat's postero-orbital sinus hair: I. Brainstem projections and the effect of infraorbital nerve section at different ages. J Comp Neurol. 1991;312:325–340. doi: 10.1002/cne.903120302. [DOI] [PubMed] [Google Scholar]


