Abstract
Both copper transporting ATPases, ATP7A and ATP7B, are expressed in mammary epithelial cells but their role in copper delivery to milk has not been clarified. We investigated the role of ATP7A in delivery of copper to milk using transgenic mice that over-express human ATP7A. In mammary gland of transgenic mice, human ATP7A protein was 10- to 20-fold higher than in control mice, and was localized to the basolateral membrane of mammary epithelial cells in lactating mice. The copper concentration in the mammary gland of transgenic dams and stomach contents of transgenic pups was significantly reduced compared to non-transgenic mice. The mRNA levels of endogenous Atp7a, Atp7b, and Ctr1 copper transporters in the mammary gland were not altered by the expression of the ATP7A transgene, and the protein levels of Atp7b and ceruloplasmin were similar in transgenic and non-transgenic mice. These data suggest that ATP7A plays a role in removing excess copper from the mammary epithelial cells rather than supplying copper to milk.
Keywords: ATP7A, ATP7B, Copper, Lactation, Mammary gland
In mammals, adequate copper supplies during early development are critical, as inadequate copper during gestation and early infancy can be fatal. Mutations of the copper efflux protein ATP7A cause the fatal disorder Menkes disease [1]. In mice, absence of the copper uptake protein Ctr1 results in embryonic lethality [2,3]. The importance of adequate supplies of copper for postnatal development is illustrated by the death of pups suckling from dams homozygous for the toxic milk mutation (tx) which results in the production of copper deficient milk [4,5]. The toxic milk phenotype is caused by a missense mutation in Atp7b [6], leading to the conclusion that this copper pump is important for copper entry into milk. The mammary epithelial cells express both copper ATPases, ATP7A and ATP7B [5,7], and as these proteins are known to export copper from cells, both are candidates for involvement in secretion of copper into milk. The export of copper from cells is mediated by the Cu-ATPases and is controlled by the process of copper-induced trafficking of the Cu-ATPases. This process is an important part of cellular and physiological copper homeostasis [8]. In cells with low copper concentrations, both ATP7A and ATP7B are localized in the transGolgi network (TGN) [9–11]. In response to rising intra-cellular copper levels, the Cu-ATPases traffic from the TGN close to or into the plasma membrane (ATP7A), or to large endosomal-like vesicles (ATP7B). In polarized cells, ATP7A has been found to traffic to the basolateral membrane [12,13], and ATP7B to subapical regions of hepatic cells [14–16]. Excess copper is then removed from cells by direct pumping across the membrane or by exocytosis [9–11].
ATP7A and ATP7B have different intracellular distribution in non-lactating and lactating mammary glands. In non-lactating mammary glands of mice, Atp7b is located in a perinuclear region [5], consistent with the transGolgi localization reported in hepatocytes [15,16]. In the lactating gland, however, Atp7b became more diffusely localized in a rather granular pattern [5]. In human non-lactating mammary gland epithelial cells, ATP7A was found in a predominantly perinuclear distribution, while lactating epithelial cells showed a cytoplasmic distribution [7]. More recently, in the rat lactating mammary gland epithelial cells, Atp7a was found through the cell, but suckling resulted in greater staining at both the basolateral and apical plasma membranes [17]. Given that the uptake of copper is increased in the mammary gland of lactating rats [18] it is possible that the relocalization of both proteins is caused by the rapid entry of copper into the epithelial cells, but lactational hormones may also play a role [17].
In order to clarify the role of ATP7A in copper delivery to milk, we have made use of transgenic mice that over-express the human Menkes protein from the chicken β-actin promoter (CAG) [19]. The mice appear normal, however, in many tissues, copper levels are lower than in non-transgenic mice [19]. Here we report the transgenic mice express approximately 10-fold more ATP7A in the mammary gland, and that the protein is primarily located on the basolateral membrane of the epithelial cells. The functional result is a reduction in the milk copper concentration consistent with a protective copper efflux role of ATP7A in the mammary epithelial cells.
Materials and methods
Animals
All animal experiments were performed in accordance with the Deakin University Animal Welfare Committee regulations, approval numbers A20/2001 and A41/2004. Non-transgenic and transgenic mice, in the C57BL/6 background, were bred and maintained on a 12-h light/12-h dark cycle, and given deionised water and standard non-purified diet (Barastoc, Ridley AgriProducts, Australia) ad libitum during pregnancy and lactation. Mammary gland, liver, brain, and kidney from dams were removed at lactation day 7 or 8, snap frozen immediately on dry ice and stored at −20 °C for metal analysis or −80 °C for subsequent evaluation of mRNA and protein expression. Stomach contents of 7- or 8-day-old pups were isolated and stored at −20 °C for metal analysis.
Screening of homozygous transgenic mice
Homozygous ATP7A transgenic mice generated by mating heterozygote males and females from line T22#2 [19] have been used for this study. Mice were genotyped to determine their zygosity status by duplex PCR of 50 ng tail DNA using two primer sets: (1) for the reference gene, endogenous Atp7a, 5′-TTACAGGAAACCTACGTATGAC and 5′-CTGTT CTCCCCTCTGTATCGC, and (2) for the transgene, ATP7A, 5′-CTGGCA AGGCAGAAGTAAGGT and 5′-GTACCAGCCTCCGAAAAACTG. Since the reference gene (Atp7a) is X-linked, a twofold increase can be detected in the linear phase of the PCR.
Antibodies
Affinity-purified ATP7A (R17-BX) and anti-Atp7b (Wnd) antibodies have been previously described [11,19]. Anti-ceruloplasmin (1:1000) was purchased from Dako. The hCTR1 antibody raised to the first 98 amino acid residues of the protein was obtained from Monty [20]. Monoclonal anti-keratin 18 was purchased from Chemicon and β-integrin was acquired from Abcam.
Western blot analysis
Mammary gland protein extracts were prepared as previously described [21]. Proteins (50 μg) were separated using precast Novex® 10% Tricine gels (Invitrogen), transferred to Hybond-C (Amersham Biosciences). Membranes were blocked in Tris-buffered saline (TBS) containing 1% skim milk and probed with R17-BX (1:2000), Atp7b (1:500), ceruloplasmin (1:1000), and keratin 18 (1:1000) primary antibodies. Protein bands were detected by peroxidase-coupled secondary antibodies (Chemicon) using Lumilight Western Blotting Substrate (Roche Diagnostics) according to the manufacturer’s instructions. Densitometry to quantify results was performed using MultiGauge version 2.2 software (Fujifilm).
Immunohistochemical analysis
Mammary gland was immersion-fixed in 4% paraformaldehyde/PBS for 2 h on ice. Equilibrated in 20% sucrose/PBS with one change overnight, embedded in OCT compound (Tissue-Tek) on dry ice and sectioned at 16 μm on a Leica CM1850 cryostat. Sections were then incubated with R17-BX (1:1000) and β-integrin (1:200); R17-BX (1:1000) and anti-Atp7b (1:4000); R17-BX (1:1000) and hCTR1 (1:500) primary antibodies. Signals were detected by incubation with fluorescently tagged secondary antibodies (Alexa 488, 594 (1:2000), Molecular Probes). Images were captured using a 40× oil objective with a LEICA TCS SP2 confocal microscope.
Real time quantitative PCR
Total RNA was isolated using the Qiagen RNeasy Mini Kit according to the manufacturer’s instructions. cDNA was generated from 5 μg RNA using StrataScript® reverse transcriptase (Stratagene) following the manufacturer’s instructions. The specific primers for ATP7A were (5′-GCTACCTTGTCA GACACGAATGAG, and 5′-TCTTGAACTGGTGTCATCCCTTT), Atp7a (5′-AAACCTTGCGAGAAGCAATTG, and 5′-GGGCAAAAGAGGTGTTTCCA), Atp7b (5′-CCTGTGTGTCTAACATAGAAAGGAGTCT, and 5′-GA CATCAAGGCGACCAACACT), Cp (5′-GAAAATATGCAAGAAAGGCA GCTT, and 5′-AACACTGTGGGAAACAAGTAGAACTCT), Ctr1 (5′-GCC TTCGTGGCAGTGTTTTT, and 5′-GCGAATGCTGACTTGAGACTTTC) and β-actin (5′-GACAGGATGGCAGAAGGAGATTACT, and 5′-T GATC CACATCTGCTGGAAGGT. Real time PCR was performed in triplicate on a AB 7500 Real Time PCR System (Applied Biosystems) coupled with SYBR Green technology as described [21].
Atomic absorption spectrophotometry
Tissues were analyzed for trace metals by air/acetylene flame or electrothermal graphite furnace atomic absorption spectrometry, using a Varian GTA 100 spectrophotometer.
Statistical analysis
All values are expressed as means ± SD, unless otherwise indicated. Student’s t-tests were used to assess statistical differences between two given genotypes using the statistical software SPSS 11. A P value < 0.05 was considered significant.
Results
Establishment of a homozygous ATP7A transgenic mice colony
A homozygous ATP7A transgenic mice colony from line T22#2 [19] was established by mating heterozygote mice which were identified by duplex PCR of tail DNA as described in Materials and methods. The homozygous T22#2 ATP7A transgenic mice, from here on referred to as ‘transgenic mice’, were subsequently maintained by brother sister mating. The homozygous male and female mice displayed no overt phenotype.
Expression of ATP7A in mammary gland tissue
The expression levels of ATP7A and the endogenous Atp7a mRNA and ATP7A protein in mammary glands of transgenic and non-transgenic lactating dams on day 7–8 of lactation, were determined by real time quantitative PCR, Western blot analysis and immunohistochemistry. ATP7A mRNA expression levels relative to endogenous Atp7a levels in the transgenic mice were increased sixfold, normalized against β-actin, which is within the range found in other tissues of ATP7A transgenic mice [19]. Western blot analysis with the R17-BX antibody, which detects both the human and murine Atp7A [19,21] revealed a 178 kDa band in mammary gland protein extracts from non-transgenic and transgenic mice, consistent with the expected molecular weight for Atp7a and ATP7A (Fig. 1) [9]. Densitometry of the Western blot showed ATP7A protein to be ~17-fold higher relative to Atp7a (normalized against a keratin 18) in the transgenic mammary gland. Keratin 18 is specifically expressed in the mammary epithelial cells [22].
Fig. 1.
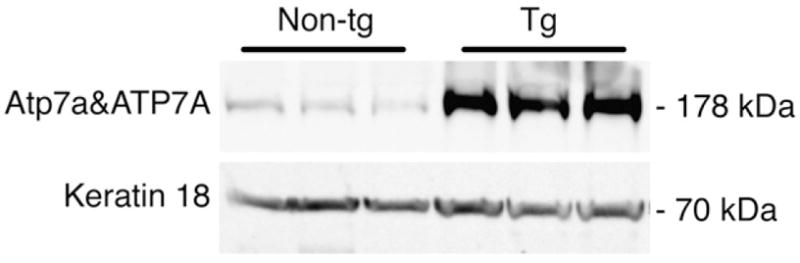
Transgene expression in the mammary gland. Representative Western blot of 50 μg mammary gland protein extracts from three non-transgenic (Non-tg) and three transgenic (Tg) females. The R17-BX antibody detected both ATP7A and Atp7a (178 kDa). Keratin 18, a protein expressed in single layer epithelial tissues was used as a loading control.
Expression of other copper-related genes
Atp7a, Atp7b, and Ctr1 mRNA levels were not significantly different in the transgenic mice compared to non-transgenic mice normalized to β-actin (Fig. 2A). However, a small but statistically significant reduction (P = 0.012) was found in Cp mRNA in transgenic mice compared to non-transgenic animals. The amount of Atp7b and Cp proteins was the same in the transgenic and non-transgenic animals (Fig. 2B). Thus, the over-expression of ATP7A does not appear to alter the expression of other copper transporter genes.
Fig. 2.
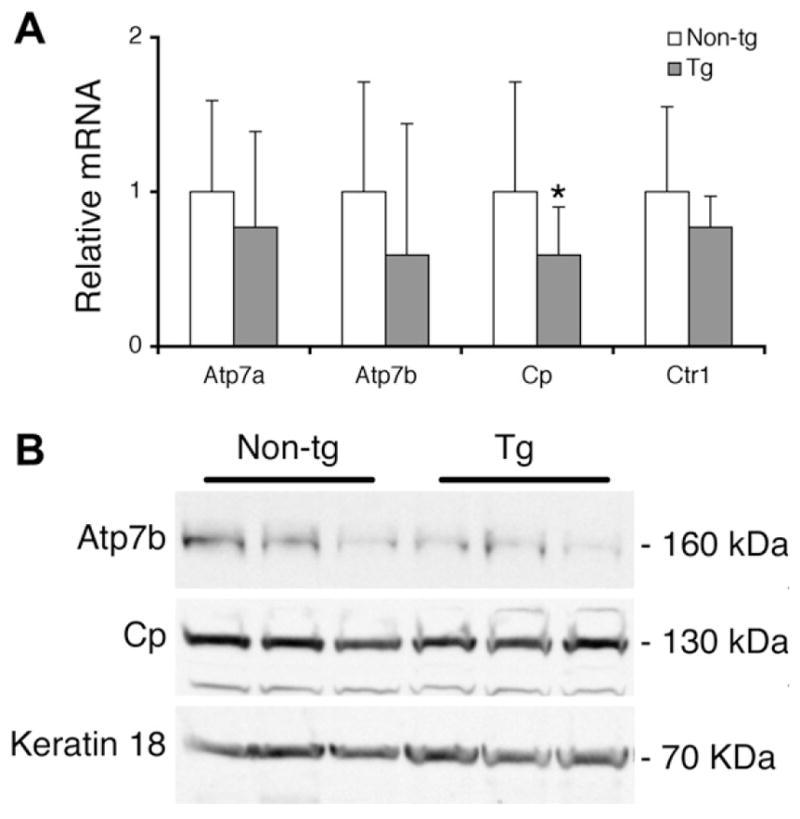
Endogenous expression of other copper-related genes. (A) Relative levels of endogenous Atp7a, Atp7b, Cp, and Ctr1 mRNA in mammary gland of transgenic (Tg) mice, n = 5 compared to non-transgenic (Non-tg) mice, n = 9 normalized against β-actin. Real time quantitative PCR data represent mean fold change ± SD from three duplicate determinations. *Significant transgene effect, P = 0.012. (B) Representative Western blots of 50 μg mammary gland protein extracts from three Non-tg and three Tg females. Keratin 18, a protein expressed in single layer epithelial tissues was used as a loading control.
Cellular localization
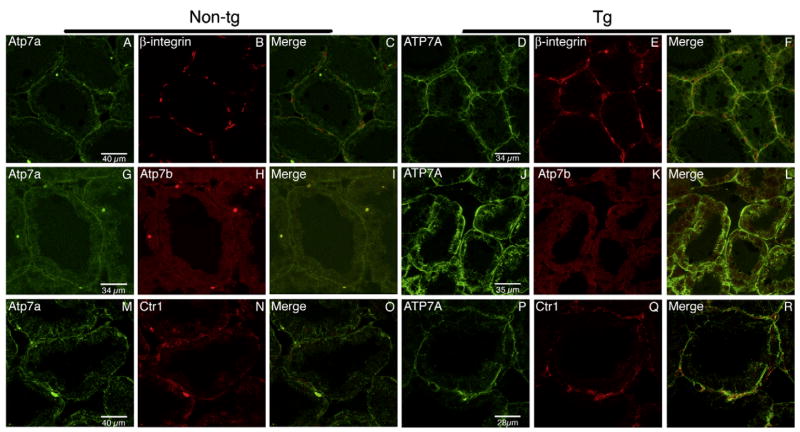
The cellular localization of Atp7a, ATP7A, Atp7b, and Ctr1 proteins in mammary gland tissue was determined by confocal microscopy (Fig. 3). In the non-transgenic mouse mammary epithelial cells, Atp7a was detected at the basolateral membrane with some cytoplasmic labeling (Fig. 3A). It also partially co-localized with β-integrin (Fig. 3B and C), an extracellular protein associated with the basolateral membrane. A similar localization but stronger signal from ATP7A was observed in the transgenic mice, more clearly demonstrating the basolateral localization (Fig. 3F). Atp7b was found to be dispersed in the cytoplasm of the lactating mammary epithelial cells in both non-transgenic and transgenic mice (Fig. 3H and K), consistent with our previous report [5] and it does not co-localize with Atp7a or ATP7A (Fig. 3I and L). Ctr1 appeared to be close to the basolateral membrane in non-transgenic mice (Fig. 3N) and transgenic mice (Fig. 3Q) where it may partially co-localize with ATP7A (Fig. 3R).
Fig. 3.
Immunofluorescence analysis of mammary gland tissue. Tissue sections were processed as described in Materials and methods and proteins were detected by indirect immunofluorescence with R17-BX (green) and β-integrin (red) (top panels), R17-BX (green) and anti-Atp7b (red) (middle panels), and R17-BX (green) and anti-hCTR1 antibodies (red) (bottom panels) using a Leica confocal microscope.
Effect of ATP7A over-expression on copper concentrations
Copper concentrations of mammary gland, liver, kidney, brain, and plasma of transgenic and non-transgenic females were determined at 7–8 day of lactation (Fig. 4). Copper concentrations were lower in the mammary gland, brain, and kidney of transgenic mice compared to non-transgenic mice (P < 0.05). The hepatic copper concentration was not reduced in these lactating transgenic mice, which is consistent with our previous finding with non-lactating females [19]. The plasma copper concentration of the transgenic females (8 ± 0.6 μmol/L) was slightly elevated relative to that of the non-transgenic female mice (7.2 ± 0.9 μmol/L), however this increase was not statistically significant (P = 0.107). The copper concentration of the stomach contents of pups suckling on transgenic dams (Fig. 4) was significantly reduced compared with those of pups from non-transgenic dams (39 ± 12 nmol/g vs. 70 ± 30 nmol/g dry wt, P < 0.001). Since the content of the pup’s stomach would have come from the milk, this indicated that the milk of the transgenic dams had less copper (Fig. 4).
Fig. 4.
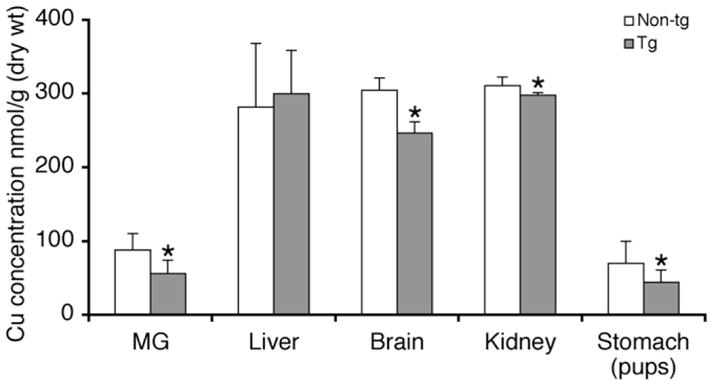
Tissue copper concentration in lactating non-transgenic and transgenic mice. Copper concentrations in tissues from non-transgenic (Non-tg), n = 9 and transgenic (Tg) mice, n = 5 were determined by atomic absorption spectroscopy. Data are means ± SD. *Significant transgene effect, P = 0.023 for mammary gland (MG), P < 0.001 for brain, P = 0.039 for kidney, and P < 0.001 for stomach contents of pups.
Discussion
In the mammary epithelial cells ATP7A was primarily associated with the basolateral membrane of the epithelial cells, but some cytoplasmic staining was evident. No ATP7A was observed at the apical surface of the cell in contrasts to the report of apical localization of the protein in lactating rat mammary gland [17]. The discrepancy could be due to the species difference, suckling stimulation, or stage of lactation. The basolateral localization suggests that ATP7A is not primarily involved in copper delivery to milk in the mouse. This conclusion is supported by the finding that the copper concentration in the stomachs of the mice suckling on the transgenic dams was 56% of that found in the pups from non-transgenic dams, indicating that the over-expression of ATP7A caused milk copper concentrations to be reduced. Moreover the copper concentration in the mammary gland of the transgenic dams was reduced by a similar extent. Over-expression of ATP7A produced a similar decrease in other tissues of the transgenic mice [19] and is consistent with enhanced efflux of copper mediated by the higher levels of ATP7A. Our results are also in agreement with the increase of copper efflux across the basolateral membrane of polarized Jeg-3 cells that over-express ATP7A [23]. Thus we suggest that these data are inconsistent with ATP7A contributing directly to copper in the milk. Rather, it would appear to be serving to remove copper from the mammary gland back into the circulation. This role would presumably protect the cells or pups from excess copper.
ATP7B appears to be the primary transporter delivering copper to milk. This conclusion is based on the reduction to 22% of normal copper observed in the milk of tx dams on day 4 of lactation [24]. The fact that the milk still contains about 25% normal copper levels suggests that a significant amount of copper reaches the milk by an ATP7B-independent mechanism. A possible source of this additional copper is the Cu–protein ceruloplasmin, which is known to be synthesized in the mammary gland and excreted in the milk [18,25]. Normally, it is thought that ceruloplasmin would receive copper in the TGN from ATP7B [26]. However, in the tx mouse this cannot be the case as the mutant Atp7b is unable to transport copper [27]. Therefore it is possible that the Menkes protein, Atp7a, is playing a role in delivering copper to Cp. This role is not inconsistent with the localization of ATP7A in the mammary gland as, despite the predominant basolateral location, there was significant cytoplasmic labeling which could suggest some ATP7A is still in the TGN. If this is the case, it would explain the puzzling results in the paper by Michalczyk et al. [5] that copper-loaded tx mice had less copper in their milk than non-copper-loaded tx mice. In this case, copper loading may have resulted in a more complete trafficking of Atp7a away from the TGN, thus further reducing the amount available for delivery to ceruloplasmin in the tx mouse.
The localization of Atp7b and ATP7A in the lactating gland agrees with previous reports of a dispersed pattern [5,7], and although clear basolateral location for ATP7A was not seen previously, it was not excluded [7]. In contrast, in the non-lactating gland, both endogenous and transgenic proteins have a tight perinuclear localisation, consistent with the TGN. The mechanism underlying the change in location in the lactating mammary is not known. One possible mechanism is that there is an increased flux of copper to the gland in response to lactation and this is sufficient to induce the trafficking of ATP7A and ATP7B. This possibility is consistent with the protective role of ATP7A proposed above. It is also consistent with the results of Donley et al. that in lactating rats, all newly absorbed copper is directed preferentially to the mammary gland where it is delivered at a higher concentration into milk [18]. Thus it is probable that there is a sufficient flux of copper into mammary epithelial cells to produce the observed trafficking of the Cu-ATPases.
An alternative mechanism is suggested by recent findings of hormonal regulation of the Cu-ATPases in placental cells by Hard-man et al. [28]. The placental choriocarcinoma line Jeg-3 expresses both ATP7A and ATP7B like mammary epithelial cells. Treatment of Jeg-3 cells with insulin and estrogen induced the trafficking of ATP7A from the TGN to the basolateral membrane and the trafficking was independent of copper. This copper-independent hormone induced trafficking suggests that a similar effect may occur in mammary epithelial cells in response to lactational hormones. Note that in placenta the roles of the two ATPases would appear to be the reverse of the mammary gland; ATP7A is responsible for the delivery of copper to the fetus across the basolateral surface of the trophoblast, but ATP7B may have a protective role in delivering copper across the apical surface to the maternal circulation. These respective roles are consistent with the changes in response to hormones.
In summary our data are consistent with distinct roles for ATP7A and ATP7B in lactation. We propose that ATP7A has a primary role in removing excess copper from the gland, and ATP7B is the main molecule responsible for delivery of copper into milk. Both proteins may have a role in delivering copper to ceruloplasmin which also provides significant copper to milk. The balance between these competing mechanisms must be regulated in some way to ensure safe delivery of adequate copper to the pups, and it is possible that this regulation involves both copper fluxes in the mammary epithelial cells as well as hormonal control.
Acknowledgments
This work was supported by the National Institutes of Health under Grant R01 HD46949-01. We thank Bi-Xia Ke for assistance with atomic absorption spectroscopy.
References
- 1.Mercer JFB. The molecular basis of copper transport diseases. Trends Mol Med. 2001;7:64–69. doi: 10.1016/s1471-4914(01)01920-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA. 2001;98:6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch H. Toxic milk, a new mutation affecting copper metabolism in the mouse. J Hered. 1983;74:141–144. doi: 10.1093/oxfordjournals.jhered.a109751. [DOI] [PubMed] [Google Scholar]
- 5.Michalczyk AA, Rieger J, Allen K, Mercer JFB, Ackland ML. Defective trafficking of the Wilson disease protein ATP7B in the mammary gland of the toxic milk mouse and the effects of copper supplementation. Biochem J. 2000;352:565–571. [PMC free article] [PubMed] [Google Scholar]
- 6.Theophilos M, Cox DW, Mercer JFB. The toxic milk mouse is a murine model of Wilson disease. Hum Mol Genet. 1996;5:1619–1624. doi: 10.1093/hmg/5.10.1619. [DOI] [PubMed] [Google Scholar]
- 7.Ackland ML, Anikijenko P, Michalczyk A, Mercer JFB. Expression of the Menkes copper-transporting ATPases, MNK, in the lactating human breast: possible role in copper transport into milk. J Histochem Cytochem. 1999;47:1553–1562. doi: 10.1177/002215549904701207. [DOI] [PubMed] [Google Scholar]
- 8.Cater MA, Mercer JFB. Copper in mammals: mechanism of homeostasis and pathophysiology. In: Tamás MJ, Martinoia E, editors. Molecular Biology of Metal Homeostasis and Detoxification. Springer-Verlag; Berlin Heidelberg, Germany: 2005. pp. 101–120. [Google Scholar]
- 9.Petris MJ, Mercer JFB, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes JR, Cox DW. Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum Mol Genet. 2000;9:1927–1935. doi: 10.1093/hmg/9.13.1927. [DOI] [PubMed] [Google Scholar]
- 11.La Fontaine S, Theophilos MB, Firth SD, Gould R, Parton RG, Mercer JFB. Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase. Hum Mol Genet. 2001;10:361–370. doi: 10.1093/hmg/10.4.361. [DOI] [PubMed] [Google Scholar]
- 12.Monty JF, Llanos RM, Mercer JF, Kramer DR. Copper exposure induces trafficking of the menkes protein in intestinal epithelium of ATP7A transgenic mice. J Nutr. 2005;135:2762–2766. doi: 10.1093/jn/135.12.2762. [DOI] [PubMed] [Google Scholar]
- 13.Greenough MA, Pase LB, Voskoboinik I, Petris MJ, Wilson-O’Brien AL, Camakaris J. Signals regulating trafficking of the Menkes (MNK; ATP7A) copper translocating P-type ATPase in polarized MDCK cells. Am J Physiol Cell Physiol. 2004;287:C1463–C 1471. doi: 10.1152/ajpcell.00179.2004. [DOI] [PubMed] [Google Scholar]
- 14.Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology. 2006;130:493–506. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol. 1999;276:G639–G646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- 16.Roelofsen H, Wolters H, Van Luyn MJA, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119:782–793. doi: 10.1053/gast.2000.17834. [DOI] [PubMed] [Google Scholar]
- 17.Kelleher SL, Lonnerdal B. Mammary gland copper transport is stimulated by prolactin through alterations in Ctr1 and Atp7A localization. Am J Physiol Regul Integr Comp Physiol. 2006;291:181–191. doi: 10.1152/ajpregu.00206.2005. [DOI] [PubMed] [Google Scholar]
- 18.Donley SA, Ilagan BJ, Rim H, Linder MC. Copper transport to mammary gland and milk during lactation in rats. Am J Physiol Endocrinol Metab. 2002;283:E667–E675. doi: 10.1152/ajpendo.00115.2002. [DOI] [PubMed] [Google Scholar]
- 19.Ke BX, Llanos RM, Wright M, Deal Y, Mercer JF. Alteration of copper physiology in mice overexpressing the human Menkes protein ATP7A. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1460–R1467. doi: 10.1152/ajpregu.00806.2005. [DOI] [PubMed] [Google Scholar]
- 20.Hardman B, Manuelpilla U, Wallace EM, Monty JF, Kramer DR, Kuo YM, Mercer JF, Ackland ML. Expression, localisation and hormone regulation of the human copper transporter hCTR1 in placenta and choriocarcinoma Jeg-3 cells. Placenta. 2006;9–10:968–977. doi: 10.1016/j.placenta.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Llanos RM, Ke BX, Wright M, Deal Y, Monty F, Kramer DR, Mercer JF. Correction of a mouse model of Menkes disease by the human Menkes gene. Biochim Biophys Acta. 2006;1762:485–493. doi: 10.1016/j.bbadis.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Taylor-Papadimitriou J, Lane EB. Keratin expression in the mammary gland. In: Neville MC, Daniel CW, editors. The Mammary Gland: Development, Regulation and Function. Plenum Press; New York: 1987. pp. 181–215. [Google Scholar]
- 23.Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer J, Ackland L. Distinct functional roles for the Menkes and Wilson copper translocating P-type ATPases in human placental cells. Cell Physiol Biochem. 2007;20:1073–1084. doi: 10.1159/000110718. [DOI] [PubMed] [Google Scholar]
- 24.Rauch H. Hepatic copper in neonatal toxic milk mice. Genetics. 1985;110:S88. [Google Scholar]
- 25.Cerveza PJ, Mehrbod F, Cotton SJ, Lomeli N, Linder MC, Fonda EG, Wickler SJ. Milk ceruloplasmin and its expression by mammary gland and liver in pigs. Arch Biochem Biophys. 2000;373:451–461. doi: 10.1006/abbi.1999.1572. [DOI] [PubMed] [Google Scholar]
- 26.Terada K, Kawarada Y, Miura N, Yasui O, Koyama K, Sugiyama T. Copper incorporation into ceruloplasmin in rat livers. Biochim Biophys Acta. 1995;1270:58–62. doi: 10.1016/0925-4439(94)00072-x. [DOI] [PubMed] [Google Scholar]
- 27.Voskoboinik I, Greenough M, La Fontaine S, Mercer JFB, Camakaris J. Functional studies on the Wilson copper P-type ATPase and toxic milk mouse mutant. Biochem Biophys Res Commun. 2001;281:966–970. doi: 10.1006/bbrc.2001.4445. [DOI] [PubMed] [Google Scholar]
- 28.Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer JF, Ackland ML. Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem J. 2007;402:241–250. doi: 10.1042/BJ20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]