Abstract
Nerve growth factor (NGF) prevents apoptosis through stimulation of the TrkA receptor protein tyrosine kinase. The downstream activation of phosphatidylinositol 3-kinase (PI 3-kinase) is essential for the inhibition of apoptosis, although this enzyme does not bind to and is not directly activated by TrkA. We have found that the addition of NGF to PC-12 cells resulted in the phosphorylation of the Grb2-associated binder-1 (Gab1) docking protein and induced the association of several SH2 domain-containing proteins, including PI 3-kinase. A substantial fraction of the total cellular PI 3-kinase activity was associated with Gab1. PC-12 cells that overexpressed Gab1 show a decreased requirement for the amount of NGF necessary to inhibit apoptosis. The expression of a Gab1 mutant that lacked the binding sites for PI 3-kinase enhanced apoptosis and diminished the protective effect of NGF. Hence, Gab1 has a major role in connecting TrkA with PI 3-kinase activation and for the promotion of cell survival by NGF.
The balance between cell death through apoptosis and the promotion of cell survival is essential for the proper formation of tissues during development and for the maintenance of mature organisms (1). The activation of receptor protein tyrosine kinases (RPTKs) by their specific ligands is a common physiologic mechanism for the prevention of apoptosis (2). For example, the neurotrophins and the Trk family of receptors have a critical role in the maturation of the nervous system (3). One well studied example is that of nerve growth factor (NGF) and its high affinity receptor, TrkA (3, 4), which have been shown to be required for the survival of sensory and sympathetic neurons (5, 6). The administration of NGF antiserum to developing animals results in the loss of sensory and sympathetic neurons (5). Similarly, mice with a homozygous deletion of either the genes for NGF or TrkA show extensive cell death in sensory and sympathetic ganglia (6).
TrkA can result in the activation of several signaling pathways including those of phospholipase C-γ, Ras/mitogen-activating protein kinase (MAPK) (7, 8), and phosphotidyinositol 3-kinase (PI 3-kinase) (9). PI 3-kinase activity appears to be essential for the antiapoptotic effect of NGF (10). This was first demonstrated in the rat pheochromocytoma cell line PC-12 (11). These cells will rapidly undergo apoptosis in serum free media (12) that can be prevented by the addition of NGF (13). The treatment of PC-12 cells with wortmannin, an inhibitor of some PI 3-kinase isozymes, renders these cells insensitive to the protective effects of NGF (10). Platelet-derived growth factor (PDGF) stimulation protects PC-12 cells transfected with the PDGF receptor from apoptosis, which requires the presence of the binding site for PI 3-kinase on the PDGF receptor (10).
PI 3-kinase isozymes that are directly activated by RPTKs and sensitive to wortmannin consist of a p85 adaptor subunit, which contains one src homology 3 (SH3) and two src homology 2 (SH2) domains, and a p110 subunit that encompasses the catalytic activity (14). While p85/110 PI 3-kinases can be phosphorylated, it is the binding of the SH2 domains that activates this enzyme (15). Unlike the PDGF receptor, TrkA does not directly bind and activate PI 3-kinase in vivo (9). This situation is reminiscent of that for the insulin and epidermal growth factor (EGF) receptors that require phosphorylation of an intermediate signaling molecule that then binds and activates PI 3-kinase. The insulin receptor phosphorylates the multisite docking protein Grb2-associated binder-1 (Gab1) (16) and insulin receptor substrates 1 (17) and 2 (18) to effect PI 3-kinase activation. The EGF receptor has been shown to phosphorylate Gab1 as well as erbB3 (19), and c-Cbl (20, 21). Such an intermediate protein has not yet been identified for TrkA (9, 22).
We have found that the addition of NGF to PC-12 cells resulted in the phosphorylation of Gab1 with the subsequent binding of several SH2 domain-containing proteins, including PI 3-kinase. By virtue of its interaction with PI 3-kinase, we have demonstrated a direct role for Gab1 in the promotion of cell survival. A significant amount of PI 3-kinase activity is associated with Gab1 during NGF signaling. PC-12 cells that overexpress Gab1 showed a decreased requirement for the amount of NGF necessary to prevent apoptosis. Expression of a Gab1 mutant lacking the binding sites for PI 3-kinase enhanced apoptosis and diminished the protective effect of NGF. These results indicate a role for Gab1 in the activation of PI 3-kinase and the promotion of cell survival by NGF.
MATERIALS AND METHODS
Cell Culture and Stable Transfections.
PC-12 cells were grown in RPMI 1640 medium containing 10% heat inactivated horse serum and 5% fetal bovine serum on Primaria tissue culture plates (Falcon). For transfections, we used the cDNA for murine Gab1 (M.H.M. and A.J.W., in preparation, and ref. 23) that is 90% identical and 98% similar to human Gab1 and retains all putative SH2 domain binding motifs. This was cloned into the mammalian expression vector pLTR2 and this construct or vector only were cotransfected with pKOneo plasmid into PC-12 cells using the Lipofectin (Life Technologies) reagent. G418 resistant clones were subcloned twice by limiting dilution. Gab1 expression was assayed by Western blot analysis.
Analysis of Gab1:PI 3-Kinase Interactions.
Immunoprecipitations, fusion protein production and Western blot analyses were performed as described (16).
Cytoplasmic DNA Preparation and Analysis.
Cytoplasmic DNAs were prepared by cell lysis in a hypotonic buffer as described (24). One-third of the DNA was electrophoresed on 1.65% agarose gels and then stained with SyBr Green (Molecular Probes). Total DNA was quantitated in a FluorImager device (Molecular Dynamics).
Site-Directed Mutagenesis.
Site-directed mutagenesis was performed using the PCR overlap-extension method (25) on a 1.2-kb PCR fragment containing all three YVPM motifs. This fragment was completely sequenced to verify that the proper Y→F mutations were introduced and no other alterations had occurred. The fragment was then ligated into the Gab1 cDNA using a PstI restriction site.
PI 3-Kinase Assays and Transient Transfections.
Immunoprecipitations against Gab1 or phosphotyrosine followed by PI 3-kinase assays were as described (16). For assays involving transient transfections, the Gab1 or Gab1ΔPI3K cDNAs were cloned in-frame and distal to the HA epitope in the pCDNA vector. Empty vector served as the control. PC-12 cells were seeded into 100-mm dishes and transfected using 12 μg of plasmid and 216 μg of the Lipofectamine reagent (Life Technologies, Grand Island, NY). Three hours following transfection, cells were fed with serum supplemented media and re-fed 24 h later. After 24 h, cells were serum starved for 12 h and mock stimulated or stimulated with NGF for 1 min prior to lysis. Immunoprecipitations were performed with anti-hemagglutinin (HA) antibody (Babco, Richmond, CA). One-tenth of the pellet was saved and used for quantitating the expression of Gab1 or Gab1ΔPI3K by Western blots with anti-HA antibody. The remainder of the pellet was used for the PI 3-kinase assay. The PIP signal was quantitated in a PhosphorImager and corrected for background using the values from the vector only controls and normalized to HA expression.
Morphologic Analysis of Apoptosis.
Cells were seeded onto coverslips coated with poly d-lysine (50 μg/ml) in six-well dishes and transfected with 2 μg of plasmid and 36 μg of Lipofectamine. Three hours after transfection cells were fed with serum containing media. For cells treated with NGF, 3 h later the cells were washed and incubated for 5 h in serum free media alone or containing NGF. Cells were then fixed and processed for immunofluorescence microscopy using fluorescein isothiocyanate-labeled secondary antibody. Coverslips were then counterstained with 4′-6-diamidino-2-phenylindole (2 μg/ml). Cells were scored for apoptosis by an independent observer.
RESULTS
NGF Stimulation Induces the Rapid Tyrosine Phosphorylation of Gab1 and the Association of SH2 Domain-Containing Proteins.
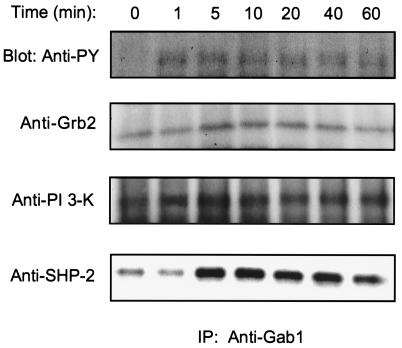
It has been noted that the stimulation of EGF and TrkA receptors in PC-12 cells can produce a similar set of early responses (26) including the activation of PI 3-kinase (27). We observed that Gab1 is present in PC-12 cells and can be phosphorylated upon EGF addition (data not shown). Because these two RPTKs may share common signaling elements, we asked if Gab1 might also be tyrosine phosphorylated following NGF stimulation. Anti-Gab1 immunoprecipitations were performed on quiescent PC-12 cells that were either mock stimulated or stimulated with NGF for various periods of time. Western blot analysis with antiphosphotyrosine antibody showed that within 1 min. of NGF addition (Fig. 1) there was a rapid tyrosine phosphorylation of Gab1 that persisted over the duration of the time course. Because Gab1 acts as a docking protein in EGF signaling, we examined if it also served to recruit SH2 domain-containing proteins during NGF signaling. Analysis of anti-Gab1 immunoprecipitations showed an association of Gab1 with PI 3-kinase and SHP-2 that was significantly enhanced by NGF stimulation and was sustained during the time periods examined. Grb2 showed NGF-induced association with Gab1, but this appeared to return to basal levels by 60 min. These results demonstrated that Gab1 is phosphorylated following NGF addition and suggested that it could play a role in the downstream signaling of NGF by the recruitment of SH2 domain-containing proteins.
Figure 1.
Gab1 is phosphorylated after NGF addition and acts as a docking protein. PC-12 cells were grown to confluence in 100-mm dishes in RPMI 1640 medium containing 5% fetal bovine serum and 10% heat inactivated horse serum. Cells were then washed once with PBS and incubated overnight in serum free media. Dishes were stimulated with NGF (100 ng/ml) for the periods of time indicated. Cell lysates (1 mg) were then used for immunoprecipitation with anti-Gab1 antibody and the Western blots incubated with either antiphosphotyrosine (Upstate Biotechnology, Lake Placid, NY), anti-Grb2, anti-PI 3-kinase, or anti-SHP-2 (all from Transduction Laboratories, Lexington, KY) antibodies.
Gab1 Binds and Activates PI 3-Kinase After NGF Stimulation.
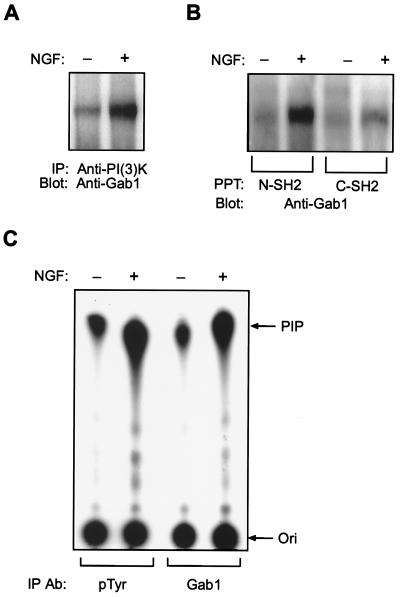
Because Gab1 can mediate PI 3-kinase activation for the EGF and insulin receptors, we sought evidence that Gab1 could bind the p85 subunit of PI 3-kinase and consequently result in enzymatic activation. Analysis of anti-p85 immunoprecipitations showed a basal association with Gab1 that was significantly increased upon NGF addition (Fig. 2A). Constitutive association of PI 3-kinase with Gab1 has been noted (16), which may be due to an SH3 domain interaction with Gab1. To verify that PI 3-kinase could interact directly with Gab1, we performed precipitations with glutathione S-transferase fusion proteins containing the SH2 domains from the p85 subunit. This revealed that both of the SH2 domains could recognize Gab1 in an NGF-dependent manner, although the N-terminal SH2 domain showed a stronger interaction (Fig. 2B).
Figure 2.
Gab1 binds PI 3-kinase and mediates its enzymatic activation during NGF signaling. (A) PI 3-kinase interacts with Gab1. PC-12 cells were serum starved and mock stimulated or stimulated with NGF as indicated. Cell lysates were used for immunoprecipitation with a polyclonal antibody against PI 3-kinase (Upstate Biotechnology) and the Western blot was incubated with anti-Gab1 antibody. (B) The SH2 domains of p85 bind Gab1. Lysates from mock stimulated (−) or NGF stimulated (+) PC-12 cells were used for precipitations with glutathione S-transferase fusion proteins containing either the N-terminal SH2 domain (N-SH2) or the C-terminal SH2 domain (C-SH2) from the p85 subunit of PI 3-kinase. The blot was incubated with anti-Gab1 antibody. (C) Gab1 mediates PI 3-kinase activation during NGF signaling. PI 3-kinase assays were performed on anti-Gab1 and antiphosphotyrosine immunoprecipitates from mock stimulated or NGF stimulated PC-12 cell lysates. PIP, position of phosphotidyinositol-3 phosphate; Ori, origin. Results shown are representative of five independent experiments.
We then examined if Gab1 was responsible for mediating PI 3-kinase activity in NGF signaling. The addition of NGF resulted in a 5.4-fold increase in association of PI 3-kinase activity in anti-Gab1 immunoprecipitations (Fig. 2C). As a measure of the contribution of Gab1 to PI 3-kinase activation by NGF, we compared the Gab1 associated activity with that found in antiphosphotyrosine immunoprecipitations. This revealed that the activity associated with Gab1 was 68% of that associated with antiphosphotyrosine, indicating that Gab1 is the major site for PI 3-kinase recruitment in PC-12 cells following NGF addition.
Overexpression of Gab1 Reduces the Concentration of NGF Necessary to Prevent Apoptosis.
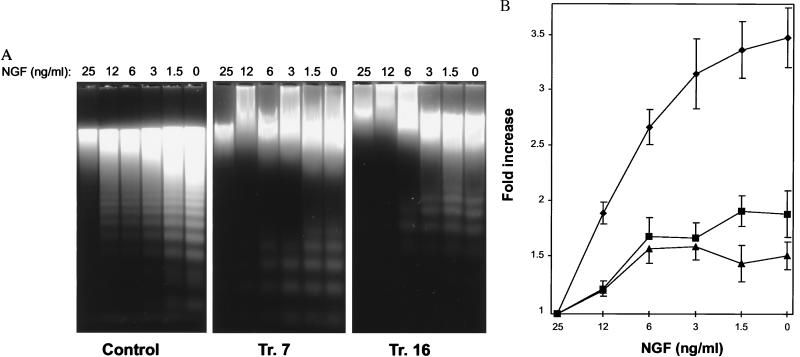
Having established that Gab1 was an important component in the activation of PI 3-kinase following NGF stimulation, we asked if Gab1 might also participate in mediating the prevention of apoptosis in PC-12 cells. We reasoned that cells overexpressing Gab1 should show a reduced requirement for the amount of NGF necessary to prevent apoptosis. We transfected the Gab1 cDNA into PC-12 cells and established two cell lines with ≈9-fold overexpression of Gab1, Tr. 7, and Tr. 16. These two cell lines and control cells were serum starved for 12 h in the presence of varying concentrations of NGF. It was found that a 25 ng/ml concentration of NGF could largely prevent apoptosis in the control cells as shown by the comparative absence of DNA fragmentation in the cytoplasmic DNA liberated from these cells. Decreasing amounts of NGF resulted in a progressive increase in the intensity of laddering (Fig. 3A). Quantitation showed that there was an ≈3.5-fold increase in the amount of cytoplasmic DNA released at the lowest concentrations of NGF (Fig. 3B). On the other hand, the two cell lines that overexpressed Gab1 did not show such a strong dependence on NGF to inhibit cell death because the comparative intensity of DNA fragmentation was decreased and there was only a ≈1.6–1.8-fold increase in cytoplasmic DNA at the lowest concentrations of NGF (Fig. 3 A and B).
Figure 3.
Overexpression of Gab1 reduces the requirement for NGF to prevent apoptosis. PC-12 cells were transfected with an expression vector containing the Gab1 cDNA. Two cell lines overexpressing Gab1, Tr. 7, and Tr. 16, and a vector-only transfectant (Control) were used. Cells were seeded in six-well dishes, grown for 2 days, then washed five times with serum free medium and incubated in serum free media containing the indicated amount of NGF or complete serum starvation. After 12 h, floating and adherent cells were harvested from which cytoplasmic DNA was prepared, electrophoresed on agarose gels and quantitated. (A) Overexpression of Gab1 enhances cell survival. Gel electrophoresis of cytoplasmic DNA from Control, Tr. 7, and Tr. 16 cell lines. (B) Quantitation of the relative amounts of cytoplasmic DNA released. Symbols are as follows: Tr. 7 (▪), Tr. 16 (▴), Control (⧫). Values shown are normalized to the DNA present in the 25 ng/ml sample and are the average of four or five independent experiments. Bars = SEM.
The pYVPM Motifs in Gab1 Mediate the Binding of PI 3-Kinase.
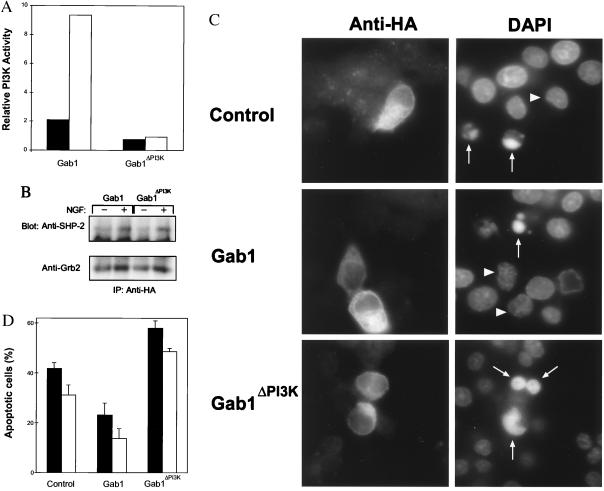
The overexpression of a Gab1 molecule that is unable to bind PI 3-kinase should diminish any protective effect of NGF. The SH2 domains of PI 3-kinase recognize the motif pYXXM (28). For example, the PDGF receptor that strongly binds and directly activates PI 3-kinase, contains the sequence YVPM and mutation from Y→F abolishes PI 3-kinase activation by PDGF (29). Because Gab1 contains three such YVPM sequences, it seemed likely that these mediated the binding and activation of PI 3-kinase. These three sites in the Gab1 cDNA were targeted for mutation from Y→F and the resulting construct was designated Gab1ΔPI3K. We first confirmed that these sites were responsible for binding PI 3-kinase by transfection of Gab1ΔPI3K into PC-12 cells. We performed transient transfections using vectors in which Gab1ΔPI3K or the wild-type cDNA were fused at the N terminus with a HA epitope tag. PI-3-kinase assays performed on anti-HA immunoprecipitations from PC-12 cells transfected with the wild-type Gab1 cDNA showed that there was a notable increase in activity following NGF stimulation (Fig. 4A). However, there was no significant change in PI 3-kinase activity associated with the Gab1ΔPI3K construct confirming that these sites are responsible for mediating the binding of PI 3-kinase. To verify that mutation of these sites did not affect the binding of Grb2 and SHP-2, we analyzed anti-HA immunoprecipitations for the association of these proteins with Gab1. This showed that there were similar amounts of these proteins bound to either wild-type or Gab1ΔPI3K following NGF addition (Fig. 4B).
Figure 4.
A Gab1 mutant lacking PI 3-kinase binding sites induces apoptosis in PC-12 cells. The Gab1 or Gab1ΔPI3K cDNAs were cloned into a pcDNA vector containing the HA epitope. These constructs or empty plasmid (Control) were transiently transfected into PC-12 cells (28). (A) Gab1ΔPI3K does not activate PI 3-kinase. PI 3-kinase assays were performed on the pellets from anti-HA immunoprecipitations and quantitated. Values were corrected for background and normalized for HA expression. Relative PI 3-kinase (PI3K) activity is shown. Solid bars, mock stimulated cells; open bars, cells treated with NGF. Results shown are representative of three different experiments. (B) Gab1ΔPI3K binds Grb2 and SHP-2 similar to wild-type Gab1. Western blots containing anti-HA immunoprecipitations were incubated with either anti-Grb2 or anti-SHP-2. (C) Gab1ΔPI3K induces apoptosis. Immunofluorescence microscopy was performed to detect cells expressing the various constructs (Anti-HA) while the nuclei were visualized using 4′-6-diamidino-2-phenylindole staining. Cells undergoing apoptosis are indicated by arrows and nuclei corresponding to the HA positive cells are designated by arrowheads, except in the case of Gab1ΔPI3K where they are co-incident. (D) Quantitation of the cells undergoing apoptosis. Cells were transiently transfected with these plasmids and then grown in serum containing media followed by growth in serum-free media or media supplemented with NGF for 5 h. The percentage of HA positive cells undergoing apoptosis is shown. The incidence of cell death observed with the control plasmid is similar to that reported by the manufacturer. Results are the average of six independent experiments. Solid bars, cells grown in serum free media; open bars, cells grown in media with NGF. Bars = SEM.
Enhancement of Apoptosis by a Gab1 Mutant Lacking the PI 3-Kinase Binding Sites.
We then studied the incidence of apoptosis in cells transfected with each of these plasmids. To directly detect cells expressing these various proteins, immunofluorescence microscopy was employed using an antibody directed against HA. To identify which cells were undergoing apoptosis, coverslips were counterstained with 4′-6-diamidino-2-phenylindole to reveal the nuclei. Cells that showed condensed or fragmented nuclei were considered apoptotic. Morphologic observations corroborated that cells expressing Gab1ΔPI3K showed an overall increase in cell death (Fig. 4C). Under conditions of serum starvation, transfection with the Gab1ΔPI3K construct produced a 39% increase in the presence of apoptotic cells as compared with that observed for the control plasmid (Fig. 4D). Significantly, when compared with cells transfected with wild-type Gab1 there was a 2.5-fold increase in apoptotic cells. The addition of NGF produced a 25% decrease in apoptotic cells in control plasmid transfected cells and a 40% reduction in cells receiving wild-type Gab1. However, the addition of NGF to Gab1ΔPI3K transfected cells resulted in only a 16% decrease in apoptotic cells indicating that the inability to bind PI 3-kinase diminished the protective effects of NGF. Similar experiments were performed using ethidium homodimer-1 to label dying cells that confirmed the results obtained with 4′-6-diamidino-2-phenylindole staining.
DISCUSSION
NGF can initiate a wide variety of cellular processes via stimulation of TrkA although this receptor is known to contain only two major autophosphorylation sites (22). The site at Y490 interacts with SHC and has been shown to be involved in RAS activation (7, 8, 30) whereas the site at Y785 binds and results in the activation of phospholipase C-γ (8). While a binding site for PI 3-kinase has been identified on TrkA (30), this site does not appear to be phosphorylated in vivo and so the mechanism by which TrkA activates PI 3-kinase has been elusive (9, 22). The discovery that Gab1 serves as a docking protein in TrkA signaling has helped to resolve how TrkA activates PI 3-kinase and may help to clarify other mechanisms by which NGF is capable of eliciting diverse effects.
Unlike the insulin receptor substrates 1 and 2 docking proteins, Gab1 lacks a phosphotyrosine binding domain but it can interact with RPTKs via two other mechanisms. It has been found in the yeast two-hybrid system that amino acids 450–532 of Gab1 can directly bind to the Met receptor (23). Alternatively, because the SH3 domains of Grb2 can bind to Gab1, Grb2 can also mediate receptor interactions (16). Recently work has shown that the efficient binding of Gab1 in vivo requires the presence of a functional Grb2 binding site on the Met receptor (31). Because Grb2 can also associate with TrkA via binding to SHC, this may account for how Gab1 interacts with TrkA.
In PC-12 cells, two important processes that NGF participates in are the promotion of differentiation and the prevention of cell death. The specific signaling pathways that result in these two phenotypes are now being delineated. It appears that the Ras/Raf/MEK/MAPK pathway is mainly responsible for differentiation. The activation of this pathway by several different RPTKs or the expression of several protooncogenes or oncogenes that impinge upon this pathway can result in differentiation (32–35). PI 3-kinase may also play a role in differentiation, although in the later stages of neurite extension (36). Although Gab1 associates with Grb2, this most likely does not play a role in differentiation because the overexpression of Gab1 can actually inhibit the activation of MAPK, probably through the formation of Grb2:Gab1 complexes at the expense of Grb2:SOS complexes (16).
In contrast, the prevention of apoptosis by NGF requires activation of PI 3-kinase and is independent of MAPK activation (10) because the expression of a Ras mutant that interferes with MAPK activation or the use of PD98059 (37), a specific inhibitor for MEK, does not affect NGF-mediated survival (38). Our understanding of this antiapoptotic pathway has recently been further expanded. PI 3-kinase activity results in the activation of two other kinases, p70S6K and AKT. p70S6K activity is not essential for the prevention of apoptosis as rapamycin, an inhibitor of this kinase, has no effect on cell survival (39, 40). On the other hand, AKT activity is directly related to the promotion of cell survival because overexpression of AKT inhibits apoptosis while dominant interfering mutants of AKT enhance cell death (40–43). With the discovery that Gab1 is the link between TrkA and PI 3-kinase activation, many of the early events in the cell survival pathway have now been defined.
Gab1 may also have a more general role in the promotion of cell survival. Gab1 is expressed in nearly all tissues of the body (16, 23) and has been shown to be downstream in the signaling pathway of several other RPTKs including the EGF, insulin (16), insulin-like growth factor 1 (M.H.M. and A.J.W., unpublished work), and Met/hepatocyte growth factor receptors (23). Interestingly, activation of these receptors by their cognate ligands have been shown to inhibit apoptosis both in vivo and in vitro (2, 39, 44, 45). For at least the EGF, insulin, and insulin-like growth factor 1 receptors, PI 3-kinase activity has been shown to be important for the antiapoptotic effect (38, 39, 45). These receptors also require phosphorylation of secondary molecules to effect PI 3-kinase activation and Gab1 has been demonstrated to be a significant site for enzymatic activation. Although the Met/hepatocyte growth factor receptor can bind PI 3-kinase directly through a single multifunctional site, Gab1 can be a major tyrosine phosphorylated substrate (23). Taken together, these facts would suggest that RPTK phosphorylation of Gab1 and the subsequent activation of PI 3-kinase may be a commonly used mechanism for the prevention of apoptosis in many cell types and physiologic situations.
Acknowledgments
We thank V. Notario and D. Martín-Zanca for reagents. This work was supported by Grants CA69495 and NS31102 from the National Institutes of Health and a grant from the American Cancer Society. M.H.M. is a recipient of a fellowship from the Ministerio de Educación y Ciencia de España.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Gab1, Grb2-associated binder-1; HA, hemagglutinin; NGF, nerve growth factor; PI 3-kinase, phosphatidylinositol 3-kinase; PDGF, platelet-derived growth factor; RPTK, receptor protein tyrosine kinase; MAPK, mitogen-activating protein kinase; SH2 and -3, src homology 2 and 3; EGF, epidermal growth factor.
References
- 1.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Collins M K L, Perkins G R, Rodríguez-Tarduchy G, Nieto M A, López-Rivas A. BioEssays. 1994;16:133–138. doi: 10.1002/bies.950160210. [DOI] [PubMed] [Google Scholar]
- 3.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 4.Barbacid M. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 5.Crowley C, Spencer SD, Nishimura M C, Chen K S, Pitts-Meek S, Armanini M P, Ling L H, MacMahon S B, Shelton D L, Levinson A D. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 6.Smeyne R J, Klein R, Schnapp A, Long L K, Bryant S, Lewin A, Lir S A, Barbacid M. Nature (London) 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 7.Stephens R M, Loeb D M, Copeland T D, Pawson T, Greene L A, Kaplan D R. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 8.Obermeier A, Bradshaw R A, Seedorf K, Choidas A, Schlessinger J, Ullrich A. EMBO J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmichi M, Decker S J, Saltiel A R. Neuron. 1992;9:769–777. doi: 10.1016/0896-6273(92)90039-g. [DOI] [PubMed] [Google Scholar]
- 10.Yao R, Cooper G M. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 11.Greene L A, Tischler A S. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batistatou A, Greene L A. J Cell Biol. 1993;122:523–532. doi: 10.1083/jcb.122.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene L A. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapeller R, Cantley L C. BioEssays. 1994;16:565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- 15.Rordorf-Nikolic T, Van Horn D J, Chen D, White M F, Backer J M. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 16.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. Nature (London) 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 17.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Nature (London) 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 18.Sun X J, Wang L M, Zhang Y, Yenush L, Myers M G, Jr, Glasheen E, Lane W S, Pierce J H, White M F. Nature (London) 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 19.Soltoff S P, Carraway K L, Prigent S A, Gullick W G, Cantley L C. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soltoff S P, Cantley L C. J Biol Chem. 1996;271:563–567. doi: 10.1074/jbc.271.1.563. [DOI] [PubMed] [Google Scholar]
- 21.Galisteo M L, Dikic I, Batzer A G, Langdon W Y, Schlessinger J. J Biol Chem. 1995;270:20242–20245. doi: 10.1074/jbc.270.35.20242. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan D R, Stephens R M. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 23.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Nature (London) 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 24.Hockenberry D, Núñez G, Milliman C, Schreiber R D, Korsmeyer S J. Nature (London) 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 25.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 26.Chao M V. Cell. 1992;68:995–997. doi: 10.1016/0092-8674(92)90068-n. [DOI] [PubMed] [Google Scholar]
- 27.Raffioni S, Bradshaw R A. Proc Natl Acad Sci USA. 1992;89:9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 29.Kazlauskas A, Cooper J A. EMBO J. 1990;9:3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obermeier A, Lammers R, Wiesmulle K H, Jung G, Schlessinger J, Ullrich A. J Biol Chem. 1993;268:22963–22966. [PubMed] [Google Scholar]
- 31.Nguyen L, Holgado-Madruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A J, Park M. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 32.Wood K W, Qi H, D’Arcangelo G, Armstrong R C, Roberts T M, Halegoua S. Proc Natl Acad Sci USA. 1993;90:5016–5020. doi: 10.1073/pnas.90.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Sagi D, Feramisco J. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 34.Cowley S, Paterson H, Kemp P, Marshall C J. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 35.Qui M S, Green S H. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 36.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. J Biol Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 37.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Párrizas M, Saltiel A R, LeRoith D. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 39.Yao R, Cooper G M. Oncogene. 1996;13:343–351. [PubMed] [Google Scholar]
- 40.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R J, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy S G, Wagner A J, Conzen SD, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 42.Kulik G, Klippel A, Weber M J. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kauffmann-Zeh A, Rodríguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Nature (London) 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 44.Ebens A, Brose K, Leonardo E D, Hanson M G, Jr, Bladt F, Birchmeier C, Barres B A, Tessier-Lavigne M. Neuron. 1996;18:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 45.Vemuri G S, McMorris F A. Development (Cambridge, UK) 1996;122:2529–2537. doi: 10.1242/dev.122.8.2529. [DOI] [PubMed] [Google Scholar]