Abstract
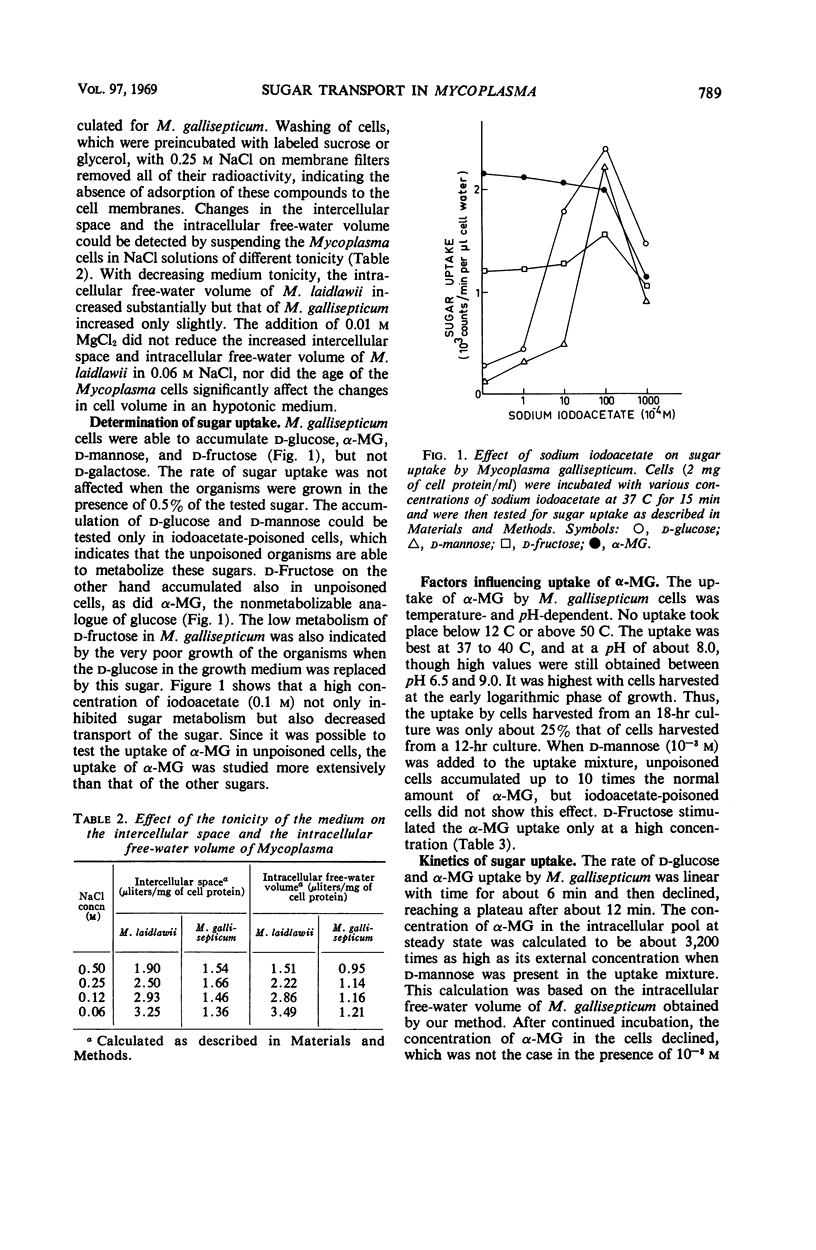
Mycoplasma gallisepticum cells were found to contain two different sugar transport systems, one for d-glucose and α-methyl-d-glucoside (α-MG) and the other for d-mannose and d-fructose. Both systems were noninducible, stereospecific, dependent on temperature and pH, and sensitive to sulfhydryl-blocking reagents. The rate of sugar uptake depended on its external concentration, obeying Michaelis-Menten kinetics. The sugar accumulated in the cells against a concentration gradient, and an energy requirement for accumulation was demonstrated with α-MG. Both transport systems thus meet the criteria of active transport. The exit of α-MG from the cells, like its entry, depended on temperature and was accelerated by energy supplied by the oxidizable d-mannose. d-Glucose accelerated α-MG exit, apparently by an exchange reaction. A method for measuring the intercellular space and intracellular free-water volume of Mycoplasma was devised, and several of its applications are described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado F. Hypothesis for the interaction of phlorizin and phloretin with membrane carriers for sugars. Biochim Biophys Acta. 1967 Jul 3;135(3):483–495. doi: 10.1016/0005-2736(67)90038-7. [DOI] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- ROBRISH S. A., MARR A. G. Location of enzymes in Azotobacteragilis. J Bacteriol. 1962 Jan;83:158–168. doi: 10.1128/jb.83.1.158-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D., YU S. H. Substrate specificity of a glucose permease of Escherichia coli. J Bacteriol. 1962 Nov;84:877–881. doi: 10.1128/jb.84.5.877-881.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Gottfried L., Rottem S. Amino acid transport in Mycoplasma. J Bacteriol. 1968 May;95(5):1685–1691. doi: 10.1128/jb.95.5.1685-1691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The cell membrane of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):115–129. doi: 10.1111/j.1749-6632.1967.tb27651.x. [DOI] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- WILKINS P. O., O'KANE D. J. ACCUMULATION OF GLUCOSE AND GALACTOSE BY STREPTOCOCCUS FAECALIS. J Gen Microbiol. 1964 Mar;34:389–399. doi: 10.1099/00221287-34-3-389. [DOI] [PubMed] [Google Scholar]



