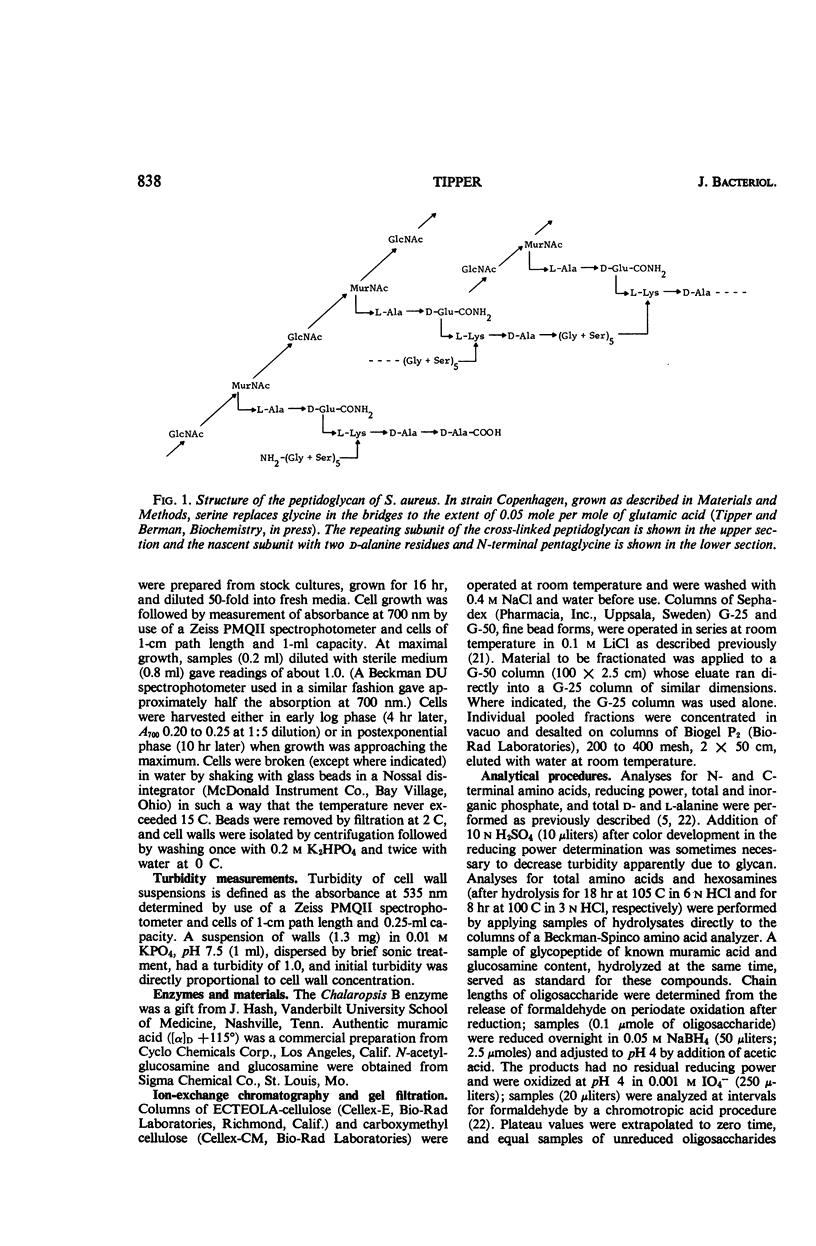
Abstract
Autolysis of isolated cell walls of Staphylococcus aureus strain Copenhagen was accompanied by the release of 1 mole of N-terminal alanine per mole of glutamic acid. No other N-terminal amino acids and no C-terminal amino acids were released. These observations indicated that complete hydrolysis of N-acetylmuramyl-l-alanine linkages (“amidase” action) had occurred. This was confirmed by fractionation and analysis of the products. Hydrolysis of 4-O-β-N-acetylglucosaminyl-N-acetylmuramic acid linkages also occurred to a variable extent; on one occasion, complete degradation to disaccharides and hexosamine-free polypeptides (with intact pentaglycine cross-bridges) occurred. In one other instance, hydrolysis within pentaglycine bridges also occurred. Analyses of intact cell walls indicated that, in vivo, glycine endopeptidase activity was negligible and amidase activity was low, but that endo-β-N-acetylglucosaminidase hydrolysed about 8% of the N-acetylglucosaminyl-N-acetylmuramic acid linkages. Autolysis of isolated cell walls was too slow for the enzymes isolated with them to have significant action during this isolation. The possible functions of these autolytic activities are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Cripps R. E., Work E. The accumulation of extracellular macromolecules by Staphylococcus aureus grown in the presence of sodium chloride and glucose. J Gen Microbiol. 1967 Oct;49(1):127–137. doi: 10.1099/00221287-49-1-127. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Lache M., Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. 3. Isolation of a new peptide dimer, N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanyl-N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanine. Biochemistry. 1968 Apr;7(4):1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- Hisatsune K., DeCourcy S. J., Jr, Mudd S. Studies on the carbohydrate-peptide fraction of the centrifugal supernatants of Staphylococcus aureus cultures. Biochemistry. 1967 Feb;6(2):586–594. doi: 10.1021/bi00854a029. [DOI] [PubMed] [Google Scholar]
- Hisatsune K., DeCourcy S. J., Jr, Mudd S. The immunologically active cell wall peptide polymer of Staphylococcus aureus. Biochemistry. 1967 Feb;6(2):595–603. doi: 10.1021/bi00854a030. [DOI] [PubMed] [Google Scholar]
- Huff E., Silverman C. S. Lysis of Staphylococcus aureus cell walls by a soluble staphylococcal enzyme. J Bacteriol. 1968 Jan;95(1):99–106. doi: 10.1128/jb.95.1.99-106.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. M. Production of lysozyme by staphylococci and its correlation with three other extracellular substances. J Bacteriol. 1966 May;91(5):1804–1810. doi: 10.1128/jb.91.5.1804-1810.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- RALSTON D. J., LIEBERMAN M., BAER B., KRUEGER A. P. Staphylococcal virolysin, a phage-induced lysin; its differentiation from the autolysis of normal cells. J Gen Physiol. 1957 May 20;40(5):791–807. doi: 10.1085/jgp.40.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of the murein. Biochem Biophys Res Commun. 1967 Sep 27;28(6):965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Leutgeb W. In vivo studies on murein synthesis. Folia Microbiol (Praha) 1967;12(3):279–282. doi: 10.1007/BF02868744. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- TIPPER D. J., STROMINGER J. L., GHUYSEN J. M. STAPHYLOLYTIC ENZYME FROM CHALAROPSIS: MECHANISM OF ACTION. Science. 1964 Nov 6;146(3645):781–782. doi: 10.1126/science.146.3645.781. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Isolation of 4-O-beta-N-acetylmuramyl-N-acetylglucosamine and 4-O-beta-N, 6-O-diacetylmuramyl-N-acetylglucosamine and the structure of the cell wall polysaccharide of Staphylococcus aureus. Biochem Biophys Res Commun. 1966 Jan 4;22(1):48–56. doi: 10.1016/0006-291x(66)90601-2. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELSCH M., SALMON J. Quelques aspects de la staphylolyse. Ann Inst Pasteur (Paris) 1950 Nov;79(5):802–813. [PubMed] [Google Scholar]
- WELSCH M. [Staphylococcal "lysozyme"]. C R Seances Soc Biol Fil. 1959;153:2080–2083. [PubMed] [Google Scholar]



