Abstract
Estrogen has been implicated in brain functions related to affective state, including hormone-related affective disorders in women. Although some reports suggest that estrogen appears to decrease vulnerability to affective disorders in certain cases, the mechanisms involved are unknown. We used the forced swim test (FST), a paradigm used to test the efficacy of antidepressants, and addressed the hypotheses that estrogen alters behavior of ovariectomized rats in the FST and the FST-induced expression of c-fos, a marker for neuronal activity, in the rat forebrain. The behaviors displayed included struggling, swimming, and immobility. One hour after the beginning of the test on day 2, the animals were perfused, and the brains were processed for c-fos immunocytochemistry. On day 1, the estradiol benzoate-treated animals spent significantly less time struggling and virtually no time in immobility and spent most of the time swimming. Control rats spent significantly more time struggling or being immobile during a comparable period. On day 2, similar behavioral patterns with still more pronounced differences were observed between estradiol benzoate and ovariectomized control groups in struggling, immobility, and swimming. Analysis of the mean number of c-fos immunoreactive cell nuclei showed a significant reduction in the estradiol benzoate versus control groups in areas of the forebrain relating to sensory, contextual, and integrative processing. Our results suggest that estrogen-induced neurochemical changes in forebrain neurons may translate into an altered behavioral output in the affective domain.
Both basic and clinical studies suggest that estrogen influences a wide range of brain regions, neuronal functions, and pathological conditions, in addition to its role in reproductive processes and behavior. One aspect of estrogen action may include an ability to alter mood and affect (1, 2). A large body of clinical literature documents the importance of estrogen in a number of hormone-related affective disorders in women, such as premenstrual dysphoric symptoms, postpartum depression, and perimenopausal and, possibly, postmenopausal depression (cf. ref. 2, for review). Estrogen replacement therapy appears to decrease vulnerability to depression in some cases and appears to be affective in augmenting the effects of conventional antidepressants, to enhance and shorten time to response, and to counter resistance to such treatment (2). Nevertheless, the efficacy of such treatment remains controversial, in part because little is known about the precise mechanism or sites of action of these therapies in the brain.
To pursue a systematic analysis of estrogen effects on behaviors and brain areas related to affective state, we used the force swim test (FST), a well-established behavioral paradigm typically used to test the efficacy of antidepressants (3). The FST consists of two sessions during which the animals are placed into water-filled cylinders, and their behaviors, i.e., struggling, swimming, and immobility (4, 5), are recorded. The utility of this test to evaluate antidepressant agents has been positive, because it has a good predictive value for their clinical effectiveness (6).
The pattern of c-fos induction in response to swim stress in the rat brain also has been elucidated by c-fos immunohistochemistry and in situ hybridization (7, 8). Immediate early genes, such as c-fos serve as a measure of neuronal activation in response to external stimuli and, therefore, indicate the circuits involved (9, 10). Additionally, activation of immediate early genes is thought to represent the initial stages in a complex long-term neuronal response leading to adaptation and plasticity (10, 11).
We addressed the hypothesis that chronic (1 week) estrogen treatment alters behavioral responses and c-fos immunoreactivity in forebrain nuclei of ovariectomized (Ovx) rats subjected to the FST. Our results indicate a significant effect of estrogen treatment on the behavior of rats subjected to the FST and on c-fos immunoreactivity in specific forebrain nuclei of those animals subjected to this test.
MATERIALS AND METHODS
Animals.
Ovx female Sprague-Dawley rats (200–250 g) (Harlan, Madison, WI) were housed 2–3 per cage and kept on a 12/12-hr light/dark cycle (lights on at 8.00 hr). Rat chow and water were available ad libitum. All animals used were at least 3 weeks postovariectomy.
Hormone Treatments.
The rats were divided into two groups and administered a single daily s.c. injection of 17β-estradiol benzoate (10 μg in 0.1 ml of sesame oil) (n = 12) or the vehicle control, sesame oil (n = 12) for 7 days. On the eighth day, the FST was performed as described below [n = 9, for estrogen-treated (E2); n = 8, for Ovx controls]. Three rats from each group were perfused without being subjected to the behavioral testing for use as controls for c-fos levels from unstressed animals as described below.
Behavior.
The FST. The FST was conducted according to the method of Porsolt et al. (3), with the exception that the behaviors were scored on the first, as well as the second, day, and that escape and swimming behaviors also were timed (4). The behavioral apparatus was a Plexiglas cylinder 45 cm high and 20 cm in diameter filled with 18 cm of water (23–25°C). Rats were placed in the cylinder for 15 min on day 1 and 5 min on day 2. After the test, the animals were placed under a heat lamp for 15 min before being returned to their cages. The sessions were videotaped, and the scoring was done by two independent observers viewing the tapes. The three behaviors scored are defined as follows: (i) struggling—rat making active attempts to escape from the tank, including visual searching for the escape routes and diving; (ii) swimming—rat staying afloat, pedaling, and making circular movements around the tank; and (iii) immobility—rat not making any active movements with, at least, one hindpaw pressed against the bottom or the wall of the cylinder. The 15-min session on day 1 was subdivided into three 5-min intervals during scoring. The interobserver and test-retest reliability values were greater than 90% for each behavior scored.
Data analysis.
For the statistical analysis systat 7.0 software (SPSS, Chicago) was used. Single-factor ANOVA with repeated measures was performed to assess the overall differences for each behavior between the Ovx control and E2 groups on day 1 of testing. Post hoc F tests were used to compare the differences in means between the groups for each behavior within each of three 5-min intervals. In most studies using the FST, day 1 is designed to acclimate the animals to the testing environment. However, our initial observations revealed significant and interesting differences between treatments. Thus, we quantified the data and used repeated-measures ANOVA to assess the multiple measures over time on individual animals. Single measures on individual animals in each group were taken on day 2; a two-tailed Student’s t test was used to assess differences between the means between groups for each behavior.
Immunocytochemistry.
Tissue preparation. One hour after the beginning of the day 2 test session, the animals were anesthetized with Nembutal and transcardially perfused with 50 ml of 0.1 M phosphate buffer (PB), pH 7.4 and 200 ml of 4% paraformaldehyde in PB. The brains were removed and postfixed for 4 hr in the same fixative before being transferred to and stored in PB at 4°C. Fifty-micrometer sections were cut by using an Oxford Vibratome, and 3–6 sections from each of eight anterior-posterior (AP) levels of interest (Bregma, +2.7, +1.0, −0.92, −1.8, −2.3, −2.8, −3.3, and −3.6; ref. 12) were processed for c-fos immunohistochemistry. The AP levels and the structures included in the analysis (see Table 1) were selected to allow the correlation of our findings with the most detailed published study of c-fos induction in the rat central nervous system in response to environmental stimuli (i.e., footshock) (13). However, the pattern of staining observed in the present study differed somewhat from that of Beck and Fibiger (13), so several additional areas were included in the cell count, such as the paraventricular nucleus of the hypothalamus, rhomboid nucleus of the thalamus, and the four subdivisions of the medial amygdaloid nucleus.
Table 1.
c-fos immunoreactivity in sampled brain areas
| Brain region | FST/Ovx | FST/E2 | Us/Ovx | Us/E2 |
|---|---|---|---|---|
| Allocortex | ||||
| Anterior cingulate cortex area 3 | 164.2 ± 10.5 | 116.0 ± 5.2* | 25.1 ± 5.7 | 19.7 ± 4.9 |
| Piriform cortex | 137.1 ± 12.9 | 128.6 ± 6.1 | 34.9 ± 5.3 | 19.8 ± 6.4* |
| Dorsopeduncular cortex | 69.9 ± 6.3 | 58.8 ± 5.2 | 7.4 ± 1.4 | 8.9 ± 1.7 |
| Infralimbic cortex | 139.0 ± 7.7 | 116.4 ± 8.8 | 51.4 ± 7.2 | 23.4 ± 2.5* |
| Lateral orbital cortex | 129.9 ± 11.1 | 77.6 ± 3.8* | 19.1 ± 4.6 | 9.6 ± 2.6* |
| Perirhinal cortex | 101.2 ± 8.6 | 88.0 ± 5.3 | 15.9 ± 1.6 | 19.6 ± 4.9 |
| Retrosplenial cortex | 182.1 ± 17.6 | 52.9 ± 5.9* | 13.3 ± 5.5 | 12.2 ± 4.3 |
| Isocortex | ||||
| Frontal cortex area 2 | 77.2 ± 11.5 | 67.9 ± 5.8 | 2.7 ± 1.2 | 5.9 ± 2.6 |
| Forelimb cortex | 143.5 ± 6.8 | 81.2 ± 12.0* | 4.3 ± 1.1 | 6.1 ± 1.9 |
| Hindlimb cortex | 133.9 ± 22.9 | 93.8 ± 14.7 | 3.1 ± 0.5 | 4.6 ± 1.3 |
| Parietal cortex area 2 | 198.1 ± 25.2 | 100.8 ± 10.5* | 19.0 ± 3.8 | 9.8 ± 2.0 |
| Temporal cortex area 3 | 136.5 ± 12.2 | 56.6 ± 4.7* | 19.4 ± 2.4 | 5.3 ± 1.0* |
| Subcortical telencephalon | ||||
| Tenia tecta | 71.4 ± 5.37 | 51.0 ± 3.7* | 11.8 ± 2.8 | 6.8 ± 2.2 |
| Anterior claustrum | 176.3 ± 4.8 | 136.5 ± 8.5* | 18.7 ± 6.2 | 22.0 ± 6.7 |
| Endopiriform n. | 78.4 ± 6.6 | 55.7 ± 3.5* | 16.0 ± 2.6 | 16.9 ± 4.6 |
| Anterior dorsomedial striatum | 45.7 ± 4.5 | 31.8 ± 3.2* | 8.3 ± 1.5 | 8.2 ± 2.1 |
| Posterior dorsomedial striatum | 41.9 ± 2.3 | 24.0 ± 3.7* | 11.0 ± 2.4 | 15.4 ± 2.2 |
| N. accumbens core | 35.7 ± 2.9 | 32.4 ± 2.7 | 3.4 ± 1.2 | 6.3 ± 1.7 |
| N. accumbens shell | 101.9 ± 8.0 | 76.6 ± 6.1* | 29.0 ± 3.8 | 30.6 ± 3.9 |
| Lateral septal n. | 163.8 ± 10.1 | 162.8 ± 12.5 | 68.9 ± 8.3 | 37.3 ± 4.1* |
| Medial septal n. | 58.1 ± 5.4 | 32.8 ± 3.2* | 16.3 ± 2.7 | 10.6 ± 2.4 |
| Bed n. stria terminalis | 54.0 ± 3.7 | 32.4 ± 3.4* | 9.7 ± 1.3 | 9.0 ± 2.2 |
| Amygdala | ||||
| Basolateral | 35.7 ± 2.3 | 24.3 ± 1.9* | 10.2 ± 2.4 | 7.1 ± 1.7 |
| Basomedial | 40.5 ± 3.8 | 43.2 ± 1.6 | 4.8 ± 1.3 | 5.7 ± 1.4 |
| Central | 12.1 ± 2.1 | 12.8 ± 2.4 | 9.8 ± 0.9 | 3.9 ± 1.1* |
| Cortical | 82.0 ± 9.2 | 80.0 ± 7.7 | 12.9 ± 1.9 | 5.9 ± 1.1* |
| Medial anterodorsal | 75.4 ± 6.4 | 39.4 ± 4.4* | 14.9 ± 3.0 | 10.9 ± 3.0 |
| Anteroventral | 26.4 ± 2.0 | 14.8 ± 1.1* | 7.4 ± 1.4 | 3.8 ± 0.7 |
| Posterodorsal | 54.5 ± 3.5 | 36.0 ± 2.8* | 10.5 ± 1.9 | 6.6 ± 2.0 |
| Posteroventral | 60.0 ± 8.4 | 43.5 ± 5.8 | 8.8 ± 1.3 | 4.6 ± 1.1* |
| Amygdalohippocampal area | 24.1 ± 2.1 | 21.3 ± 1.4 | 3.8 ± 0.8 | 2.5 ± 0.6 |
| Hippocampus dorsal CA1 | 22.4 ± 1.9 | 8.3 ± 1.5* | 1.3 ± 0.4 | 0.78 ± 0.4 |
| Dorsal CA3 | 16.3 ± 2.0 | 7.3 ± 0.9* | 2.9 ± 0.8 | 3.1 ± 1.7 |
| Dorsal dentate gyrus | 17.6 ± 1.7 | 19.0 ± 2.9 | 5.9 ± 0.6 | 7.2 ± 0.9 |
| Thalamus | ||||
| Centromedian n. anterior | 76.2 ± 6.2 | 33.4 ± 2.9* | 19.8 ± 3.1 | 10.1 ± 2.5* |
| Posterior | 84.4 ± 11.7 | 16.5 ± 2.0* | 2.8 ± 0.8 | 5.8 ± 1.8 |
| Lateral habenula | 34.7 ± 3.7 | 35.8 ± 2.8 | 9.3 ± 1.5 | 3.1 ± 1.0* |
| Paraventricular n. anterior | 138.1 ± 8.7 | 85.4 ± 9.5* | 55.4 ± 13.4 | 43.5 ± 10.4 |
| Posterior | 125.3 ± 6.6 | 104.1 ± 4.3* | 17.8 ± 4.0 | 12.9 ± 1.6 |
| Rhomboid n. | 89.8 ± 8.6 | 32.6 ± 2.8* | 19.4 ± 4.3 | 11.8 ± 3.5 |
| Hypothalamus | ||||
| Paraventricular n. | 192.9 ± 11.7 | 85.4 ± 9.5* | 11.6 ± 3.1 | 7.4 ± 1.4 |
| Lateral n. | 36.7 ± 3.3 | 22.6 ± 3.0* | 7.1 ± 1.8 | 4.8 ± 0.6 |
| Ventromedial n. | 43.7 ± 7.8 | 10.1 ± 1.6* | 4.6 ± 1.8 | 5.2 ± 0.5 |
| Dorsomedial n. | 93.1 ± 5.9 | 47.4 ± 4.0* | 13.3 ± 2.9 | 11.1 ± 2.1 |
| Dorsal hypothalamic area | 74.1 ± 3.1 | 66.0 ± 4.3 | 13.6 ± 1.5 | 7.4 ± 1.7* |
Numbers of c-fos immunoreactive cell nuclei (mean ± SEM) in brain areas from Ovx control and Ovx E2 rats subjected to the FST (FST/Ovx and FST/E2, respectively) and in the same areas from the control and E2 animals not subjected to the FST (Us/Ovx and Us/E2, respectively). ∗ indicate significant differences (P < 0.05) between the two hormonal conditions in each case.
Immunocytochemistry protocol.
Immunocytochemistry was performed as described (14). Briefly, after permeabilization with 0.3% Triton X-100 in 0.8% BSA-PBS, sections were incubated in primary antibody to c-fos (Oncogene) in incubation buffer [1% normal donkey serum (NDS), 0.3% Triton X-100 in BSA-PBS] at a dilution of 1:15,000 or in incubation buffer alone, as a control overnight at 4°C. The sections then were washed in PBS and incubated in biotinylated donkey anti-rabbit IgG (Amersham) diluted 1:200 in 1% NDS, BSA-PBS in the cold for 60 min. After the washes, sections were incubated with streptavidin biotinylated horseradish peroxidase complex (Amersham) diluted 1:100 in PBS for 30 min in the cold. The sections were washed, and the reaction product was visualized by adding 0.05% diaminobenzidine-4-HCl (Pierce) and 0.01% H2O2 for 3 min. The sections were washed with PB, dried, and mounted on slides.
Data analysis.
For counting of the immunoreactive cell nuclei, 3–4 sections per each brain area were analyzed. The 0.17-mm2 area (0.395 mm × 0.430 mm) from structures of interest were digitized by using a MTI (Michigan City, IN) charge-coupled device digital camera mounted on Olympus Vanox-T microscope at ×50 magnification. Each digitized image was individually set at a threshold to subtract the background optical density, and the numbers of cell nuclei above the background were counted by using the computer-based image analysis system inquiry (Loats Associates, Westminster, MD). F tests of variance were run on numbers of immunoreactive cell nuclei from individual brain regions from experimental and control conditions. That value determined whether t tests for equal or unequal variance were performed to compare the cell counts from individual brain regions of control and experimental conditions. P < 0.05 was defined as the level of significance between groups.
RESULTS
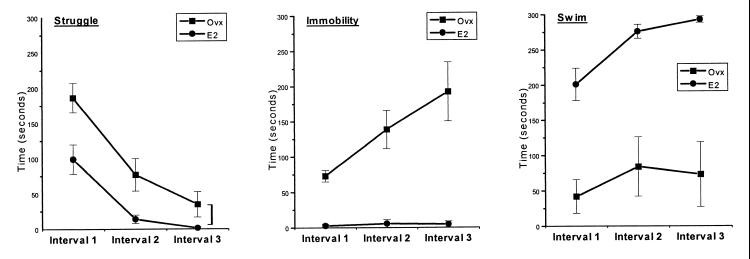
The 15-min swim interval on day 1 of the FST was divided into three 5-min intervals during which the three behaviors were recorded. The pattern and duration of behaviors clearly varied throughout the 15-min testing session on day 1, with struggling being the dominant response during the initial 5-min period (Fig. 1). The single-factor ANOVA with repeated measures revealed significant differences for all three behaviors between the E2-treated and control groups. E2 animals spent consistently less time struggling [F(1/15) = 8.22, P < 0.05], exhibited very little immobility throughout the test, unlike the Ovx rats [F(1/15) = 33.18, P < 0.05], and also spent significantly more time swimming as compared with Ovx controls [F(1/15) = 25.29, P < 0.05].
Figure 1.
FST (day 1). Each behavior (struggle, immobility, and swim) was observed and timed during three 5-min intervals of a 15-min trial. Each point represents the mean of the time the animals in each group spent in each activity/posture. Ovx control animals spent significantly (P < 0.05) more time struggling than those given estrogen (E2). Virtually none of the E2 animals spent time in immobility, whereas Ovx animals increased their time in immobility as their struggle time decreased. E2 animals spent significantly (P < 0.05) more time swimming than the Ovx controls. In general, Ovx animals displayed considerably more variability in their behaviors, evidenced by greater SEM bars.
Differences between the groups in mean time (in sec) spent in struggling reached statistical significance (as determined by post hoc F tests) for interval 1 [F(1/15) = 7.20, P < 0.05] and interval 2 [F(1/15) = 6.06, P < 0.05], but not for interval 3 [F(1/15) = 3.92, P > 0.05]. With a decrease in struggling in the Ovx group, there was a concomitant increase in immobility. On the other hand, animals in the E2 group spent virtually no time struggling during a comparable period. Differences between the two groups in mean time spent in immobility during all three intervals were statistically significant (post hoc F tests): interval 1 [F(1/15) = 61.38, P < 0.05], interval 2 [F(1/15) = 19.67, P < 0.05], and interval 3 [F(/15) = 22.32, P < 0.05]. Throughout the test session, E2 rats spent more time swimming than the Ovx controls. Differences between the groups in mean time spent swimming during all three intervals were statistically significant (post hoc F tests): interval 1 [F(1/15) = 19.31, P < 0.05], interval 2 [F(1/15) = 16.38, P < 0.05], and interval 3 [F(1/15) = 25.88, P < 0.05].
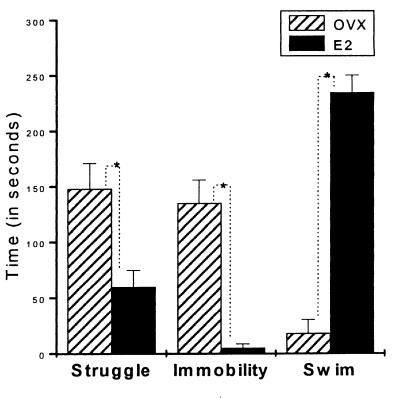
On day 2, the behavioral differences between the groups persisted, with the more rapid acquisition of the final response, i.e., swimming for the E2 and immobility for the Ovx control rats (Fig. 2). As on day 1, estrogen treatment significantly (t test) reduced the struggle time (P < 0.05) and immobility time (P < 0.05) and resulted in increased swimming time (P < 0.05).
Figure 2.
FST (day 2). Each behavior was observed for 5 min. Bars represent the mean of the time the animals spent in each activity/posture. Ovx rats displayed significantly more struggling and immobility than those in the E2 group. E2 rats spent 20 times more time swimming than the Ovx controls. The variance in behavior for the Ovx animals has decreased, possibly caused by learning experience from the first day.
In animals subjected to the FST, 45 areas of the forebrain were assayed for c-fos imunoreactivity after the FST in Ovx female rats injected either with estradiol benzoate or vehicle control. C-fos expression was induced in animals subjected to the FST in many of the regions examined. In the majority of brain structures, the number of c-fos immunopositive cell nuclei was lower in the estradiol benzoate-treated group as compared with the Ovx controls, with 29 of 45 structures showing a statistically significant reduction (Table 1). No brain area examined showed a significant increase in c-fos immunoreactivity in the E2 group.
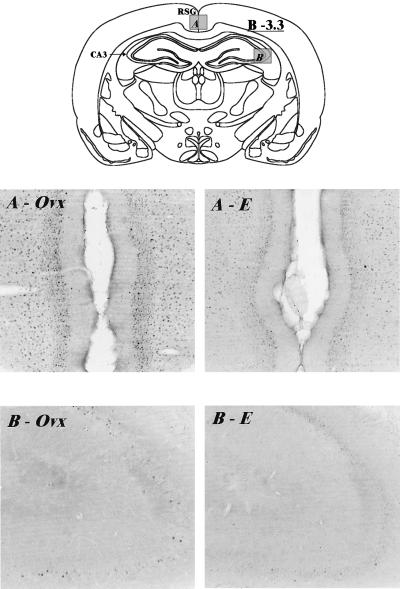
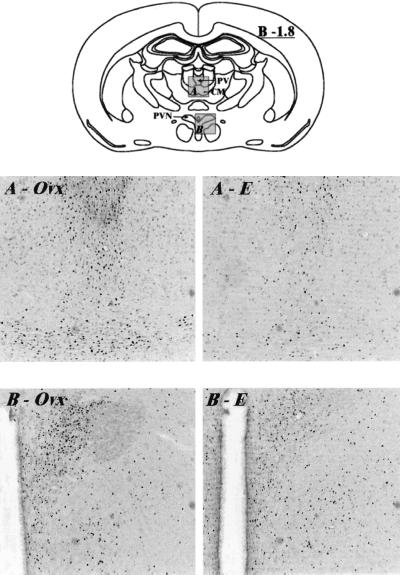
A reduction in c-fos immunolabeling with estrogen treatment relative to Ovx controls in the retrosplenial cingulate gyrus and CA3 region of the hippocampus is illustrated in Fig. 3, for example. Similarly, Fig. 4 shows a reduction in c-fos immunoreactivity in cellular nuclei of the paraventricular and centromedial thalamic nuclei and paraventricular hypothalamic nucleus in Ovx animals treated with estrogen compared with Ovx controls. Several notable structures, however, showed no difference in response to estradiol benzoate treatment, including lateral septum, piriform cortex, basomedial amygdala, central amygdala, and dorsal hippocampal dentate gyrus.
Figure 3.
Digitized images of c-fos immunoreactive cell nuclei taken from the retrosplenial cingulate gyrus (area A on the schematic) and the CA3 region of the hippocampus (area B on the schematic) of Ovx and E2 rats subjected to the FST. A illustrates the reduction in c-fos immunolabeling observed in the retrosplenial cingulate gyrus in the E2 rat (A–E) compared with the Ovx (A-Ovx) animal. Similar differences between hormonal conditions are seen in the CA3 region of the hippocampus in B. Bregma level −3.3 (12). Magnification: ×35. The diagrams used here and in Fig. 4 were taken from the Rat Brain Clipart Package (Neurographics, Kanata, Ontario, Canada).
Figure 4.
Digitized images of c-fos immunoreactive cell nuclei taken from the midline thalamus (area A on the schematic) and the paraventricular hypothalamic nucleus (area B on the schematic) of Ovx and E2 rats subjected to the FST. Arrows on the schematic indicate the paraventricular (PV) and centromedian (CM) thalamic nuclei, and the paraventrular hypothalamic nucleus (PVN). A demonstrates the reduction in c-fos immunolabeling seen in the PV and CM in the E2 group (A–E) compared with the Ovx controls (A-Ovx). Similar differences between hormonal conditions were observed in the PVN in B. Bregma level, −1.8 (12). Magnification: ×35.
The control animals not subjected to the FST were transported to the test room together with animals subjected to the FST, but placed in a different corner of the room away from the testing area. The c-fos data from these animals served to isolate the effects of the test from the stress of the transport and the novel visual and olfactory environment of the test room. The data from the unstressed (Us) E2 and Ovx control groups (Us/E2 and Us/Ovx, respectively) (both n = 3) are summarized in Table 1. As compared with the data from the animals subjected to the FST (FST/E2 and FST/Ovx), the numbers of c-fos immunoreactive cells in the control groups are much lower in most brain regions (Table 1). Certain areas, however, i.e., lateral septum, still expressed measurable amounts of c-fos. Eleven brain areas in the estradiol benzoate-treated group also showed a significant reduction in the number of labeled cells compared with Ovx controls. The most pronounced reductions in areas showing a significant c-fos induction were observed in piriform cortex (76%), infralimbic cortex (219%), and lateral septum (85%).
DISCUSSION
The present study shows that estrogen significantly altered the behavior of Ovx rats subjected to the FST. Quantitative analysis of c-fos immunolabeling in the forebrains of these animals indicated that estrogen effected a widespread reduction in the expression of this immediate early gene in many of the areas examined.
Behavioral observations indicate that estrogen treatment imparted a qualitatively different pattern of response to acute stress. In Ovx control rats, the initial overriding stress-activated escape (struggle) behavior, with time, was replaced by the initially less dominant reactive behavior of immobility. In the E2 group, however, the significantly shorter initial struggling response was followed by swimming behavior. The difference in response was evident on the first day of testing: by the third 5-min interval on day 1, the two groups already were displaying their characteristic responses, i.e., swimming for the E2 group and immobility for the Ovx controls.
On day 2, a more dramatic behavioral dichotomy with more rapid acquisition of behavioral differences was observed. Exposure of rats to the first swim session on day 1 appeared to decrease the latency for the display of group-specific behaviors on day 2. This observation is consistent with the findings of Armario et al. (4), showing the time course of responses by the animal to the FST after 3 days of testing. One possibility is that animals learned the nature of the test conditions and upon the repeated encounter with the same stressor displayed the strategy they developed during the initial exposure.
The surprising magnitude of the difference in responses between the experimental and control groups led us to consider several possible reasons for such differences in the behavioral profiles of the two groups. For example, the documented effects of estrogen on endothelial nitric oxide production (15) suggests a scenario whereby this gas neuromessenger, acting as cerebrovascular dilator, may couple brain activity to blood flow, thereby globally increasing neuronal activity (16). As another example, estrogen effects on locomotor behavior (17) also may be involved, but they are unlikely to provide the sole basis for the observed effects. First, the Ovx/control and E2 groups exhibited distinct behavioral profiles. Second, despite the observed increase in swimming in the estradiol benzoate-treated group, the longer duration of struggling (which may be more related to attempts to escape than is swimming; ref. 4) in the Ovx controls argues against the locomotor effects of estrogen being the major contributor to the observed behavioral disparities. Moreover, estrogen influenced c-fos immunoreactivity in areas related to affective behavior, such as the amygdala and hippocampus. Possible estrogenic effects on swimming and locomotor activity could be compared with the diagnostic criteria for affective disorders. Estrogen has been shown to have an antidepressant-like effect (i.e., improvement of mood) in certain cases (2), and psychomotor retardation is one of several of such criteria for major depressive episodes (cf. ref. 13, for discussion).
A modification of the original procedure used in the present study was to subdivide the active behaviors into struggling and swimming. Studies have shown that these two behaviors are differentially affected by serotonergic (i.e., serotonin specific reuptake inhibitors [SSRIs]) and adrenergic antidepressants (5, 13). Although the adrenergic agents, like desipramine, selectively prolong the struggle time without affecting swimming (4), the SSRIs selectively enhance the swimming component (5), precisely the effect observed in the present study in the E2 group. It is possible that estrogen influences in this behavioral model may be indirect, through its action on the serotonergic system, for example, as estrogenic effects on this system are well documented (e.g., cf. refs. 18–20). Moreover, estrogen has been shown to augment serotonergic function in postmenopausal women (2).
Our finding of an estrogen effect on immobility time is in agreement with a recent report showing that long-term administration of low doses of estradiol benzoate reduced that behavior (21). In another study, 17β-estradiol reversed gonadectomy-induced lengthening in immobility in mice subjected to the FST in both males and females (22). In cycling rats, immobility increased during diestrous and decreased during estrous (23, 24). In addition, in this study it was shown that inescapable shocks increased immobility only during diestrous (24). These results further support the notion of an ameliorating effect of estrogen on stress-related behaviors.
The immunocytochemical data demonstrate the overall reduction of c-fos induction in response to swim stress in E2 rats as compared with the Ovx controls. The immediate early gene c-fos is a marker of neuronal activity and has been shown to be induced by a variety of conditional and unconditional stimuli in the rodent brain (7, 8, 13, 25, 26). Taken together, such studies have established a detailed map of the stress-responsive brain circuit. Because several of these studies used swimming as a stressor, we were able to directly assess the consistency of our findings concerning the spatial distribution and relative magnitude of the response (7, 8). The observed neuroanatomical pattern of c-fos immunoreactivity was in agreement with the aforementioned studies, reflecting the general activation of the sensory input-responsive brain circuit. The important finding of this study was the ability of estrogen to down-regulate the responsivity of selected portions of this circuit. Estrogen treatment resulted in the statistically significant down-regulation of c-fos immunoreactivity in 29 of 45 (64%) of the structures examined, with none of the areas showing a significant increase. The observed effects extended well beyond the “classical” estrogen-binding areas. Although it is unclear as to whether or not some of the observed effects of estrogen are direct or indirect, the global nature of its action on c-fos immunoreactivity suggests a much broader area of influence for this hormone than previously thought.
Overall, the effect of estrogen treatment on c-fos induction bears a striking resemblance to that seen after chronic treatment with antidepressants. That is, a chronic course of antidepressant treatment (13, 27), as well as chronic electroconvulsive seizure (28), has resulted in down-regulation of c-fos mRNA in the rat frontal cortex in response to acute stress.
ABBREVIATIONS
- FST
forced swim test
- Ovx
ovariectomized
- E2
estrogen-treated
- Us
unstressed
References
- 1.Fink G, Summer B E, Rosie R, Grace O, Quinn J P. Cell Mol Neurobiol. 1986;16:325–344. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halbreich U. Neurology. 1997;48:S16–S20. doi: 10.1212/wnl.48.5_suppl_7.16s. [DOI] [PubMed] [Google Scholar]
- 3.Porsolt R D, Anton G, Blavet N, Jalfre M. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 4.Armario A, Gavald B A, Marti O. Eur J Pharmacol. 1988;158:207–212. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- 5.Detke M J, Rickels M, Lucki I. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 6.Willner P. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 7.Cullinan W E, Herman J P, Battaglia D F, Akil H, Watson S J. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 8.Duncan G E, Johnson K B, Breese G R. J Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagar S M, Sharp F R, Curran T. Science. 1988;204:128–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 10.Morgan J I, Curran T. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 11.Curran T, Franza B R., Jr Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos S G, Watson C. The Rat Brain in Sterotaxic Coordinates. New York: Academic; 1982. [Google Scholar]
- 13.Beck C H, Fibiger H C. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachman I M, Pfaff D W, Cohen R S. Brain Res. 1996;740:291–306. doi: 10.1016/s0006-8993(96)00901-8. [DOI] [PubMed] [Google Scholar]
- 15.Geary G G, Krause D N, Duckles S P. Am J Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C. Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- 17.Gerall A A, Napoli A M, Cooper U C. Physiol Behav. 1973;10:225–229. doi: 10.1016/0031-9384(73)90302-8. [DOI] [PubMed] [Google Scholar]
- 18.Biegon A, Fischette C T, Rainbow T C, McEwen B S. Neuroendocrinology. 1982;35:287–291. doi: 10.1159/000123396. [DOI] [PubMed] [Google Scholar]
- 19.Biegon A, Reches A, Snyder L, McEwen B S. Life Sci. 1983;32:2015–2021. doi: 10.1016/0024-3205(83)90053-x. [DOI] [PubMed] [Google Scholar]
- 20.Sumner B E, Fink G. Neurosci Lett. 1997;234:7–10. doi: 10.1016/s0304-3940(97)00651-4. [DOI] [PubMed] [Google Scholar]
- 21.Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Jpn J Pharmacol. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- 22.Hilakivi-Clarke L. J Stud Alcohol. 1996;57:162–170. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- 23.Marvan M L, Chavez-Chavez L, Santana S. Arch Med Res. 1996;27:83–86. [PubMed] [Google Scholar]
- 24.Marvan M L, Santana S, Chavez-Chavez L, Bertran M. Arch Med Res. 1997;28:369–372. [PubMed] [Google Scholar]
- 25.Campeau S, Hayward M D, Hope B T, Rosen J B, Nestler E J, Davis M. Brain Res. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- 26.Pezzone M A, Lee W S, Hoffman G E, Rabin B S. Brain Res. 1992;597:41–50. doi: 10.1016/0006-8993(92)91503-7. [DOI] [PubMed] [Google Scholar]
- 27.Morinobu S, Nibuya M, Duman R S. Neuropsychopharmacology. 1995;12:221–228. doi: 10.1016/0893-133X(94)00067-A. [DOI] [PubMed] [Google Scholar]
- 28.Winston S M, Hayward M D, Nestler E J, Duman R S. J Neurochem. 1990;54:1920–1925. doi: 10.1111/j.1471-4159.1990.tb04892.x. [DOI] [PubMed] [Google Scholar]