Abstract
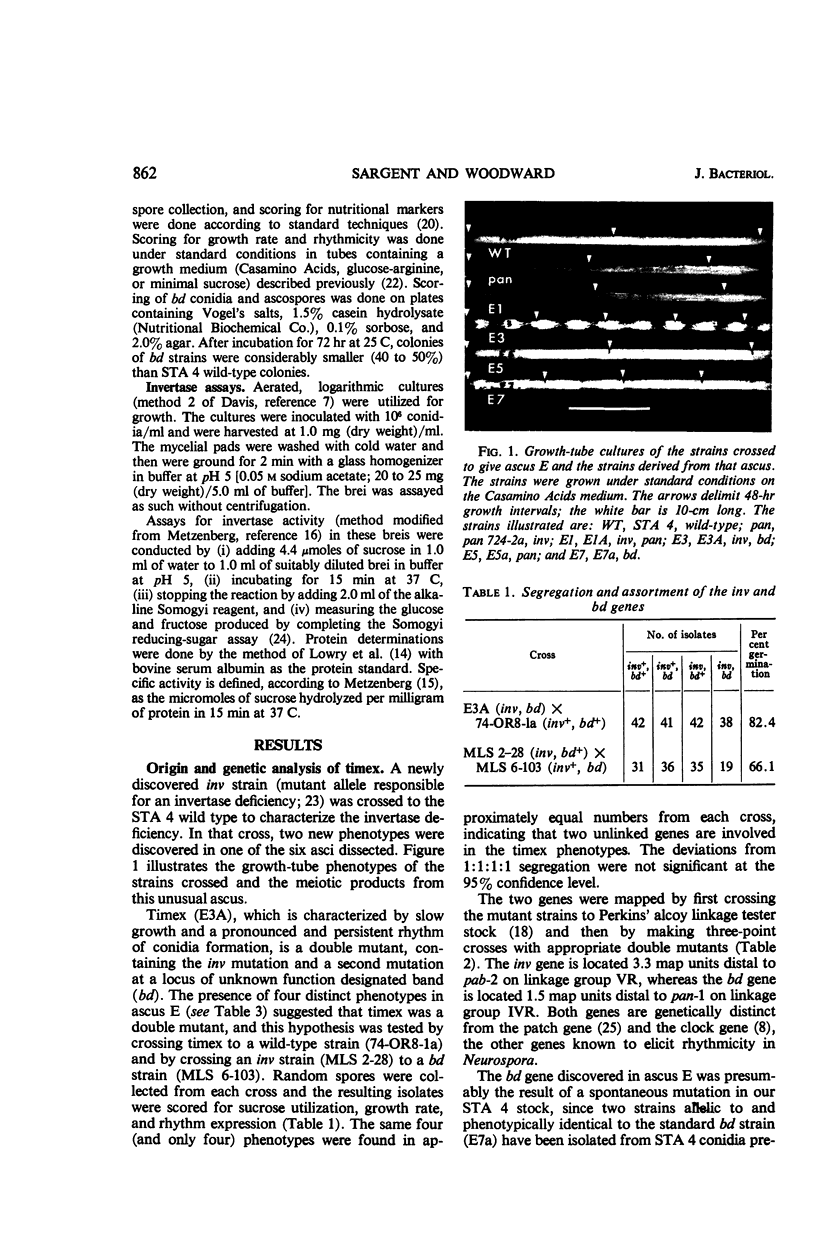
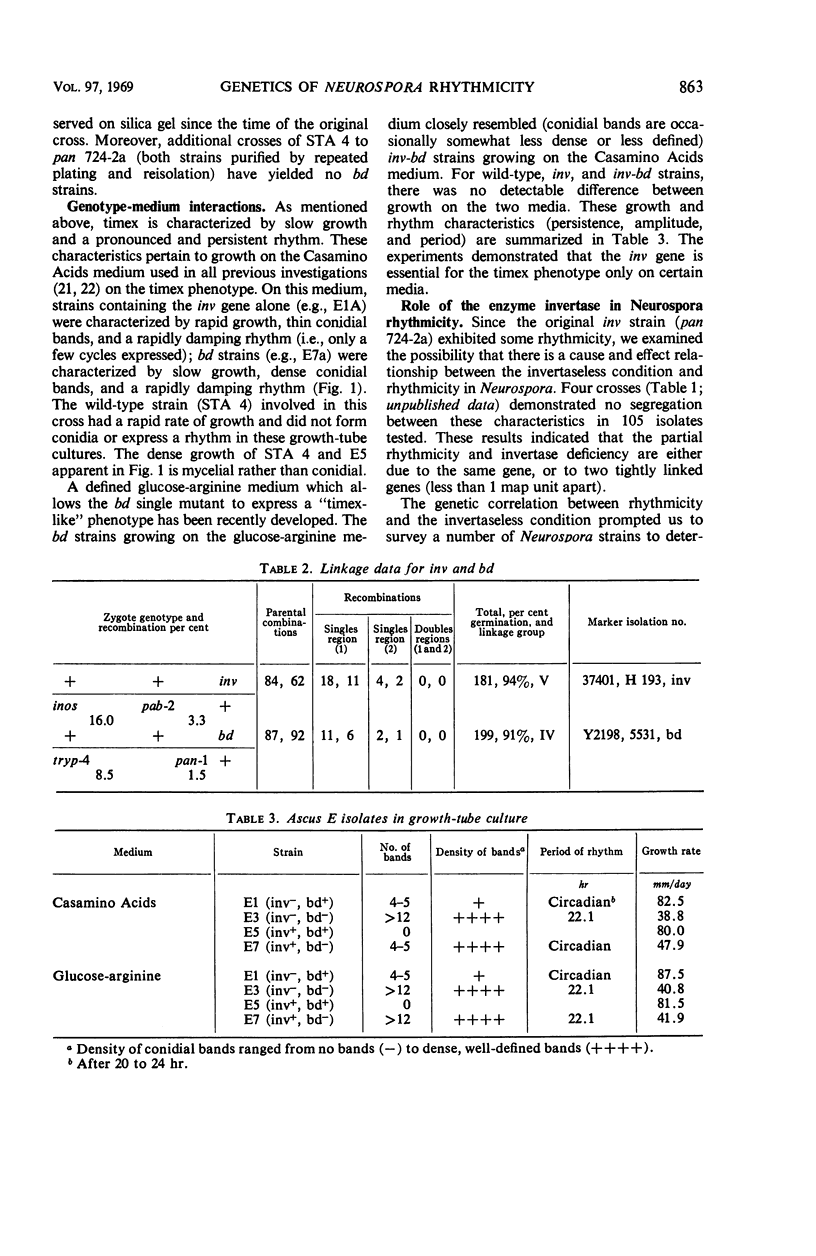
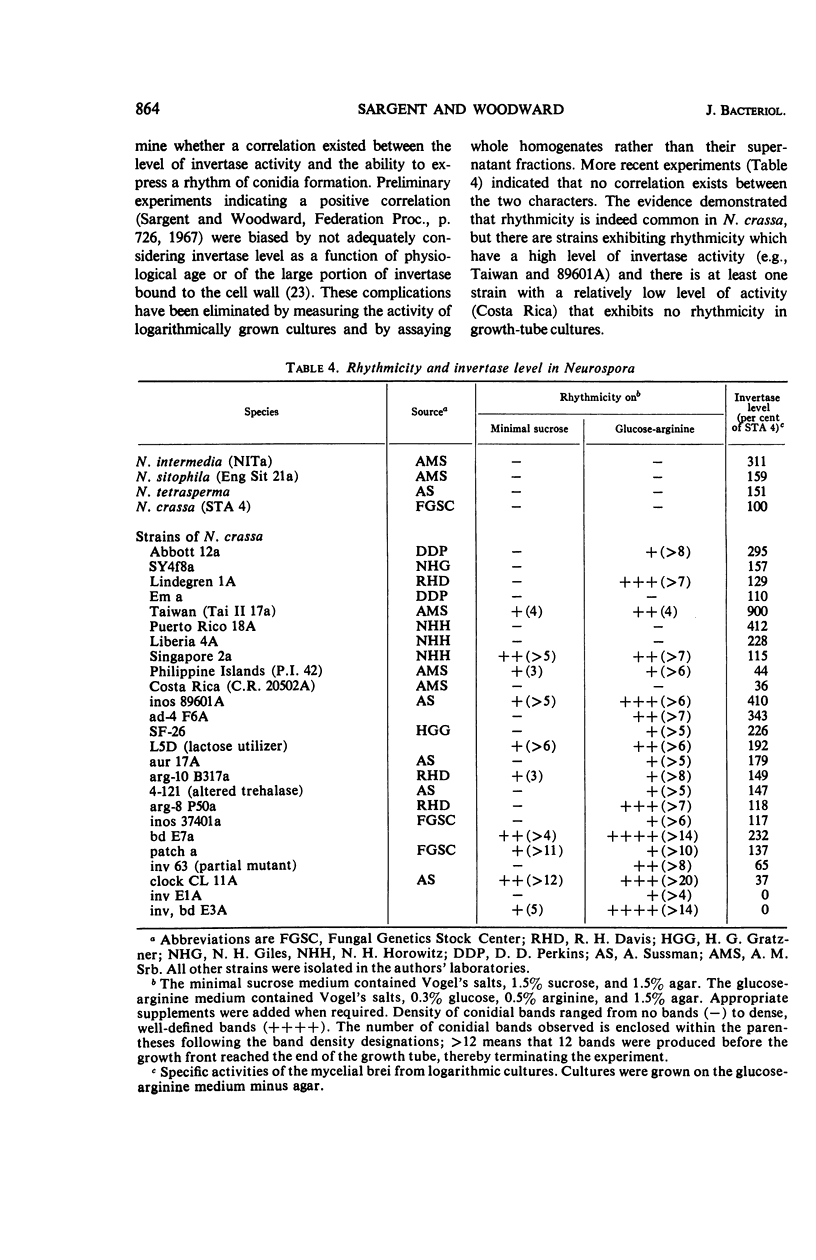
Timex, a strain of Neurospora crassa which exhibits a circadian rhythm of conidia formation in growth-tube cultures, has been found to differ from wild-type strains by two genes. One gene, inv, is responsible for an invertase deficiency, whereas the second gene, bd, is of unknown function. Both genes map independently from other genes known to induce Neurospora rhythmicity. The inv gene is not essential for the timex phenotype because bd strains express that phenotype on certain media. Although inv strains do exhibit some rhythmicity of their own, the rhythmicity apparently is not a direct result of the invertase deficiency, since there is no correlation between invertase level and rhymicity in 29 strains tested. Of the 29 strains tested, 20 exhibited some rhythmicity in growth-tube cultures, suggesting that morphological manifestations of rhythmicity in Neurospora may result from the function or the loss of function of numerous genes, or both. There was no correlation in these strains between rhythmicity and (i) genetic background; (ii) geographical origin; or (iii) nutritional requirements.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCHOFF J. Comparative physiology: diurnal rhythms. Annu Rev Physiol. 1963;25:581–600. doi: 10.1146/annurev.ph.25.030163.003053. [DOI] [PubMed] [Google Scholar]
- Durkee T. L., Sussman A. S., Lowry R. J. Genetic localization of the clock mutant and a gene modifying its band-size in Neurospora. Genetics. 1966 Jun;53(6):1167–1175. doi: 10.1093/genetics/53.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret C. F., Trucco E. Molecular models for the circadian clock. I. The chronon concept. J Theor Biol. 1967 May;15(2):240–262. doi: 10.1016/0022-5193(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Feldman J. F. Lengthening the period of a biological clock in Euglena by cycloheximide, an inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1080–1087. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAKASHIAN M. W., HASTINGS J. W. THE EFFECTS OF INHIBITORS OF MACROMOLECULAR BIOSYNTHESIS UPON THE PERSISTENT RHYTHM OF LUMINESCENCE IN GONYAULAX. J Gen Physiol. 1963 Sep;47:1–12. doi: 10.1085/jgp.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R. The effects of light on a circadian rhythm of conidiation in neurospora. Plant Physiol. 1967 Nov;42(11):1504–1510. doi: 10.1104/pp.42.11.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R., Woodward D. O. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966 Oct;41(8):1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Gene-enzyme relationships in Neurospora invertase. J Bacteriol. 1969 Feb;97(2):867–872. doi: 10.1128/jb.97.2.867-872.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman A. S., Durkee T. L., Lowry R. J. A model for rhythmic and temperature-independent growth in 'clock' mutants of Neurospora. Mycopathol Mycol Appl. 1965 Apr 14;25(3):381–396. doi: 10.1007/BF02049924. [DOI] [PubMed] [Google Scholar]




