Abstract
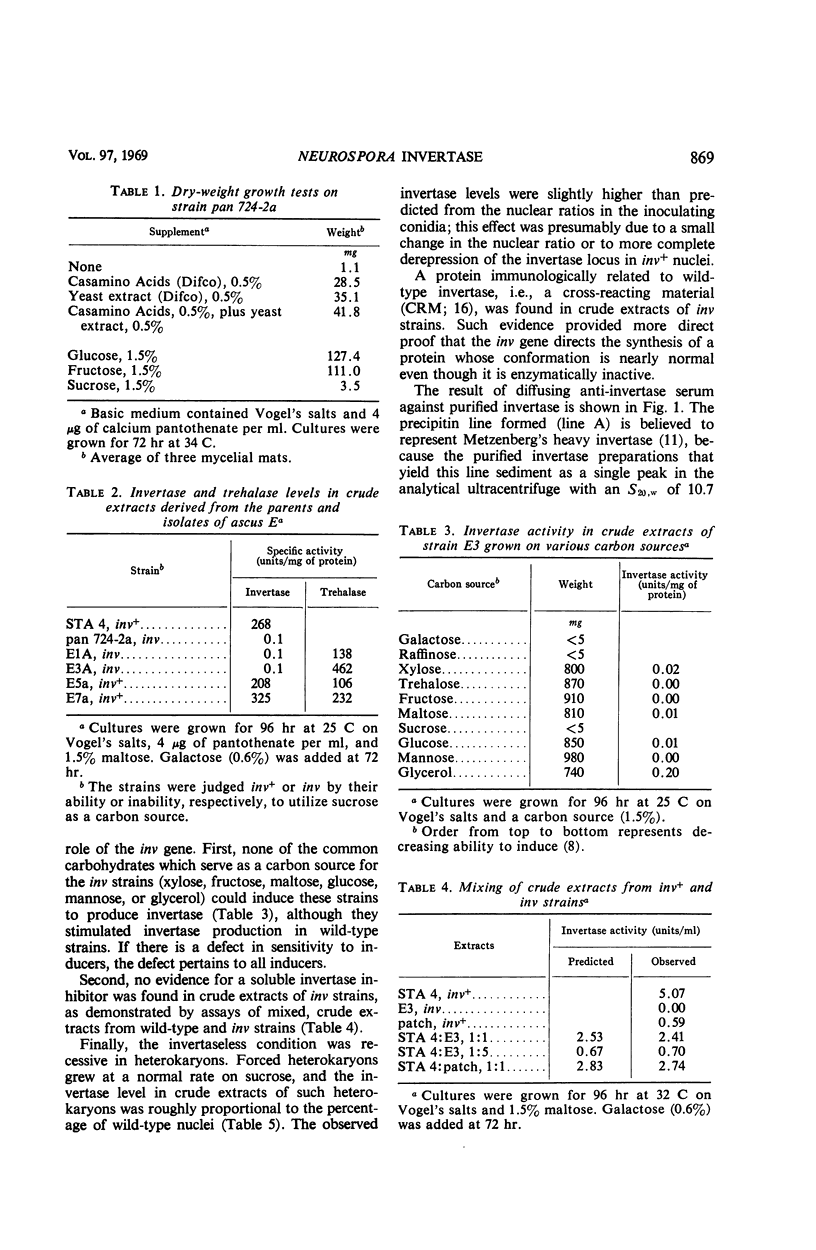
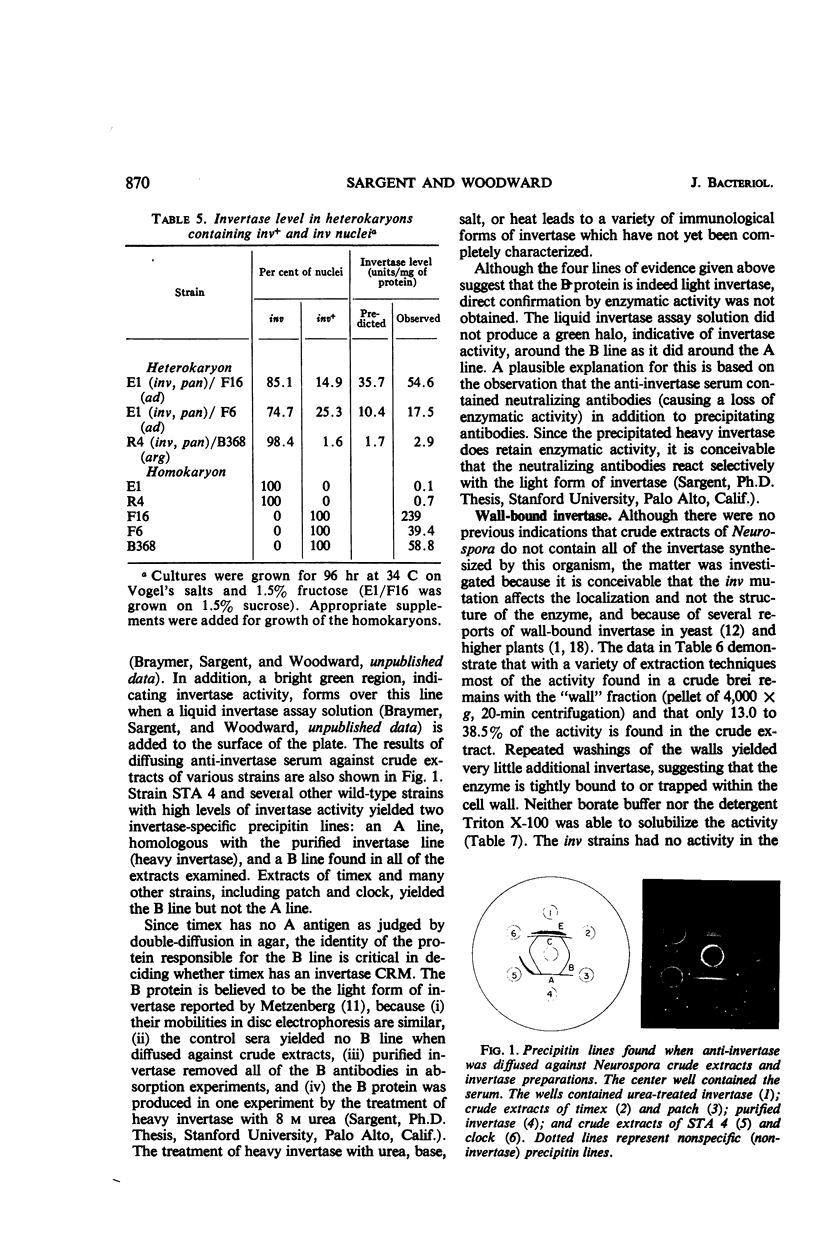
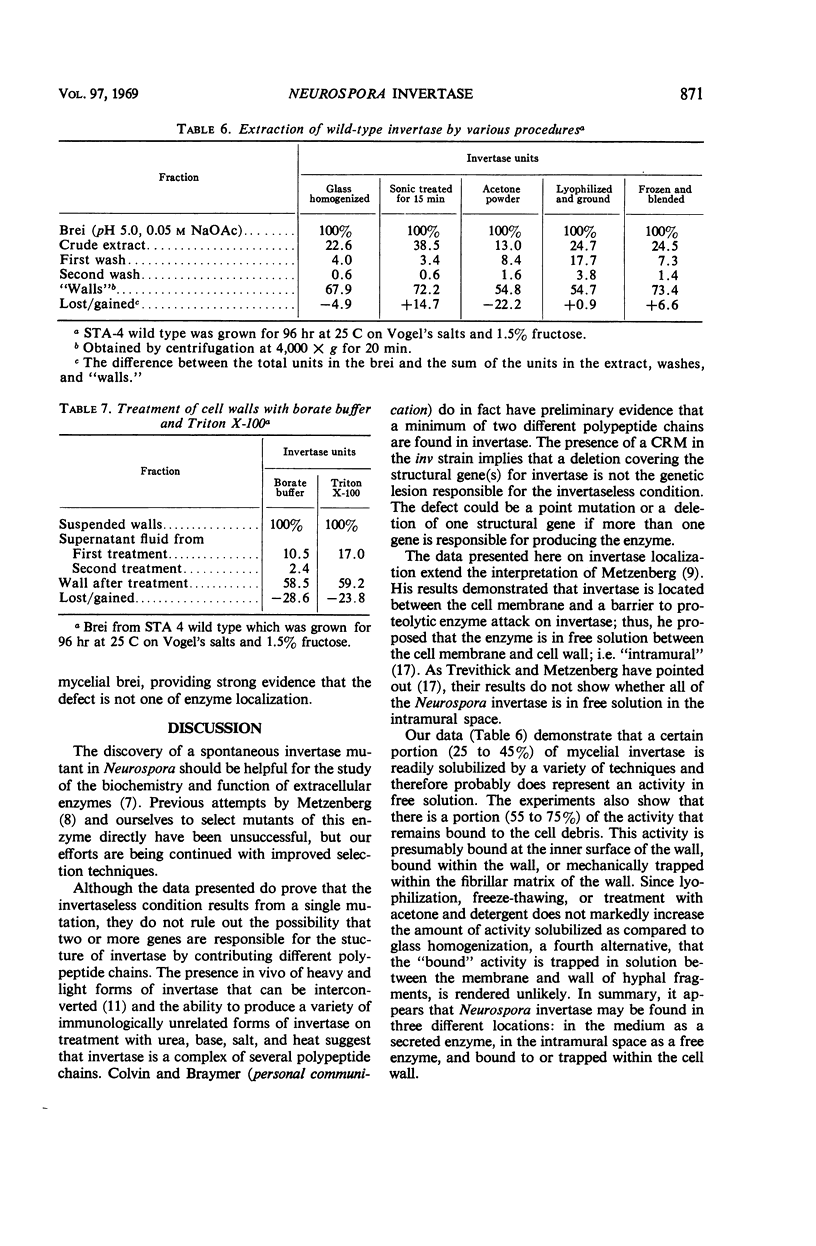
A spontaneous, single-gene mutation responsible for a total lack of invertase activity in Neurospora crassa is described. The mutation is believed to lie in the structural gene for invertase, since an immunologically cross-reacting protein is made by the mutant strain. In addition, there was no evidence for a defect in regulation of invertase activity or synthesis by the following criteria. (i) The invertaseless condition was recessive in heterokaryons; (ii) no invertase inhibitor was found in mutant extracts by mixing experiments; and (iii) none of the several sugars able to induce activity in wild-type strains was able to induce activity in the mutant strain. It was also discovered that most of the wild-type enzyme (55 to 75%) cannot be washed free from the rapidly sedimenting cell debris. This finding provided additional support for the hypothesis that Neurospora invertase is located within or about the cell wall.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. Beta-fructofuranosidase from grape berries. II. Solubilization of a bound fraction. Biochim Biophys Acta. 1966 Oct 17;128(1):124–129. doi: 10.1016/0926-6593(66)90148-2. [DOI] [PubMed] [Google Scholar]
- Arnold W. N. Beta-fructofuranosidase from grape berries. Biochim Biophys Acta. 1965 Oct 25;110(1):134–147. doi: 10.1016/s0926-6593(65)80102-3. [DOI] [PubMed] [Google Scholar]
- Atwood K C, Mukai F. Nuclear Distribution in Conidia of Neurospora Heterokaryons. Genetics. 1955 Jul;40(4):438–443. doi: 10.1093/genetics/40.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. PURIFICATION AND PROPERTIES OF TREHALASE (S) FROM NEUROSPORA. Arch Biochem Biophys. 1963 Sep;102:389–396. doi: 10.1016/0003-9861(63)90246-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. ENZYMICALLY ACTIVE SUBUNITS OF NEUROSPORA INVERTASE. Biochim Biophys Acta. 1964 Aug 26;89:291–302. doi: 10.1016/0926-6569(64)90217-2. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. The purification and properties of invertase of Neurospora. Arch Biochem Biophys. 1963 Mar;100:503–511. doi: 10.1016/0003-9861(63)90118-8. [DOI] [PubMed] [Google Scholar]
- MYRBACK K. Studies on yeast invertase; soluble and insoluble invertase (saccharase) of baker's yeast. Arch Biochem Biophys. 1957 Jul;69:138–148. doi: 10.1016/0003-9861(57)90481-2. [DOI] [PubMed] [Google Scholar]
- Prout T, Huebschman C, Levene H, Ryan F J. The Proportions of Nuclear Types in Neurospora Heterocaryons as Determined by Plating Conidia. Genetics. 1953 Sep;38(5):518–529. doi: 10.1093/genetics/38.5.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Genetic determinants of circadian rhythmicity in Neurospora. J Bacteriol. 1969 Feb;97(2):861–866. doi: 10.1128/jb.97.2.861-866.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskind S. R., Yanofsky C., Bonner D. M. ALLELIC STRAINS OF Neurospora LACKING TRYPTOPHAN SYNTHETASE: A PRELIMINARY IMMUNOCHEMICAL CHARACTERIZATION. Proc Natl Acad Sci U S A. 1955 Aug 15;41(8):577–582. doi: 10.1073/pnas.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Molecular sieving by Neurospora cell walls during secretion of invertase isozymes. J Bacteriol. 1966 Oct;92(4):1010–1015. doi: 10.1128/jb.92.4.1010-1015.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D., Macdonald I. R. Development of soluble and insoluble invertase activity in washed storage tissue slices. Plant Physiol. 1967 Mar;42(3):456–458. doi: 10.1104/pp.42.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]




