Abstract
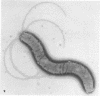
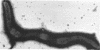
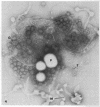
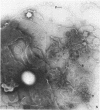
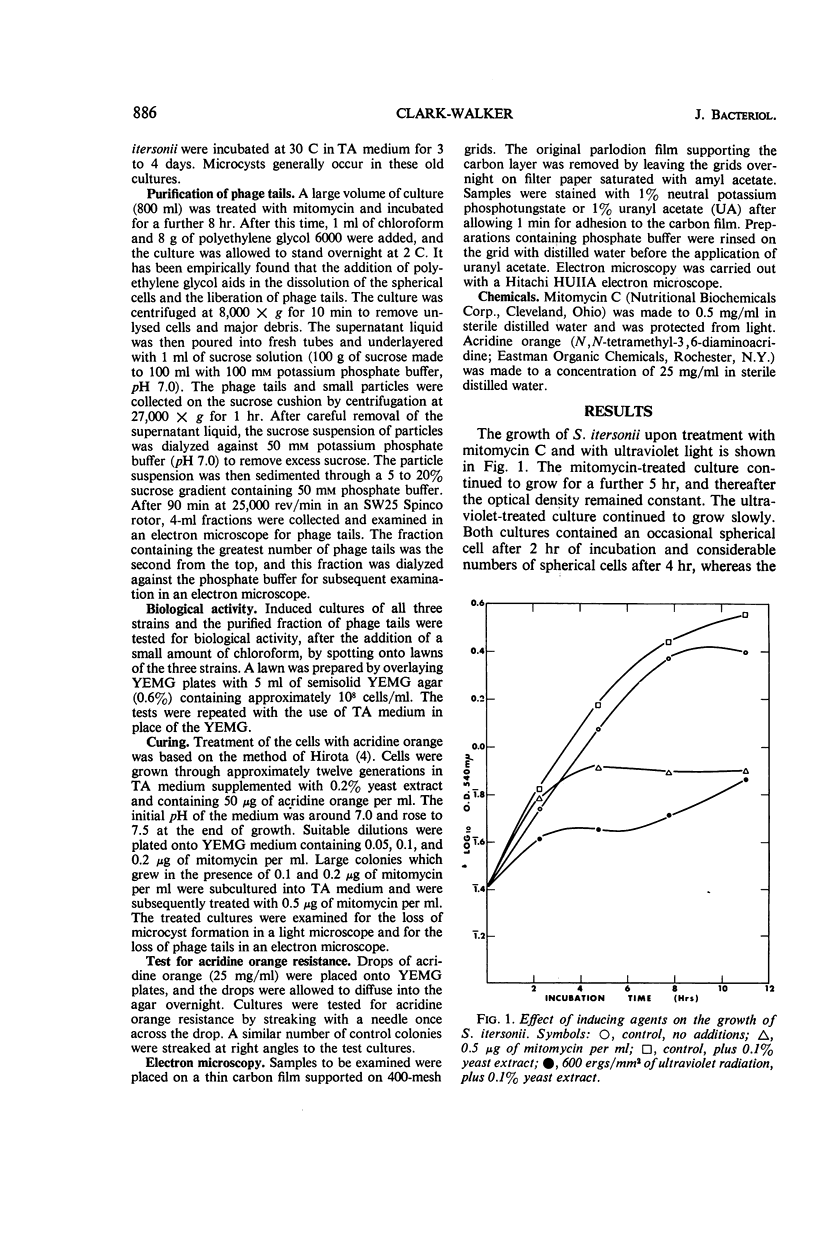
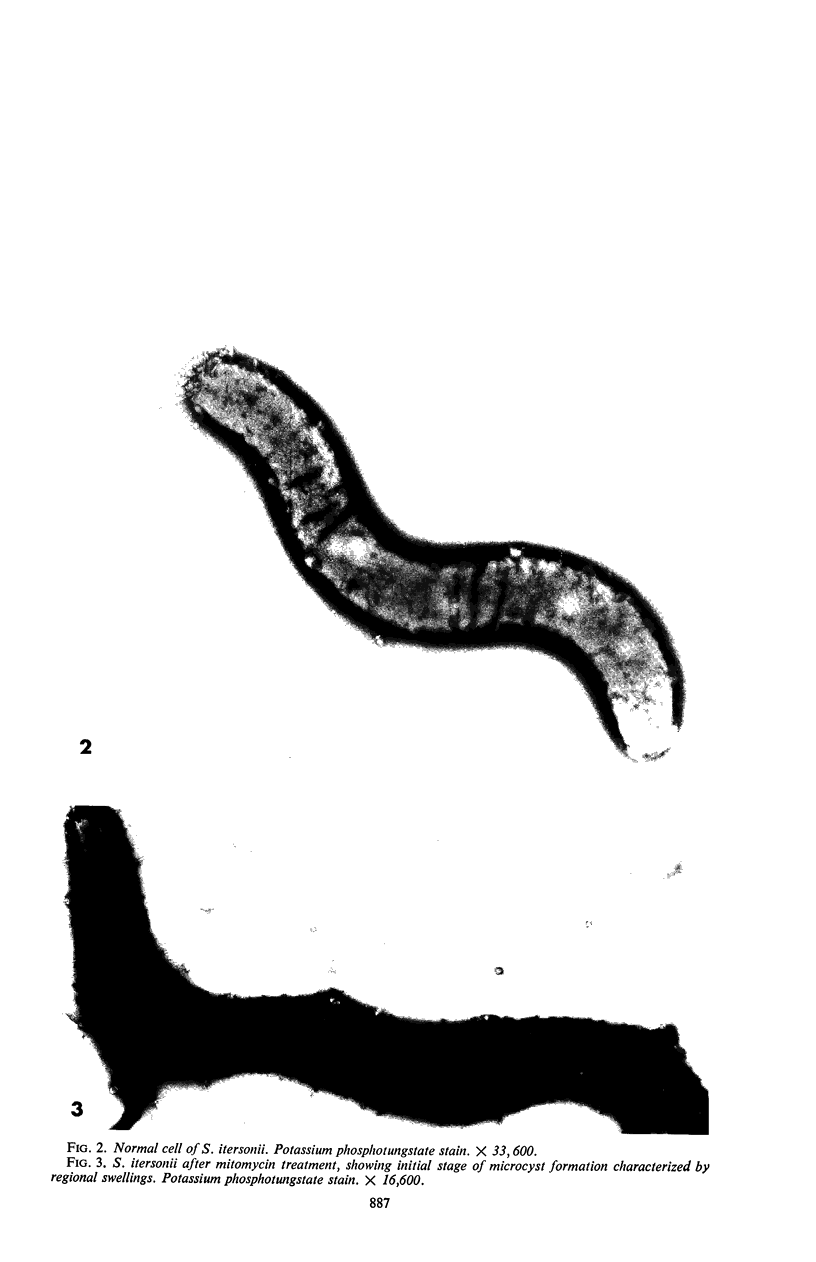
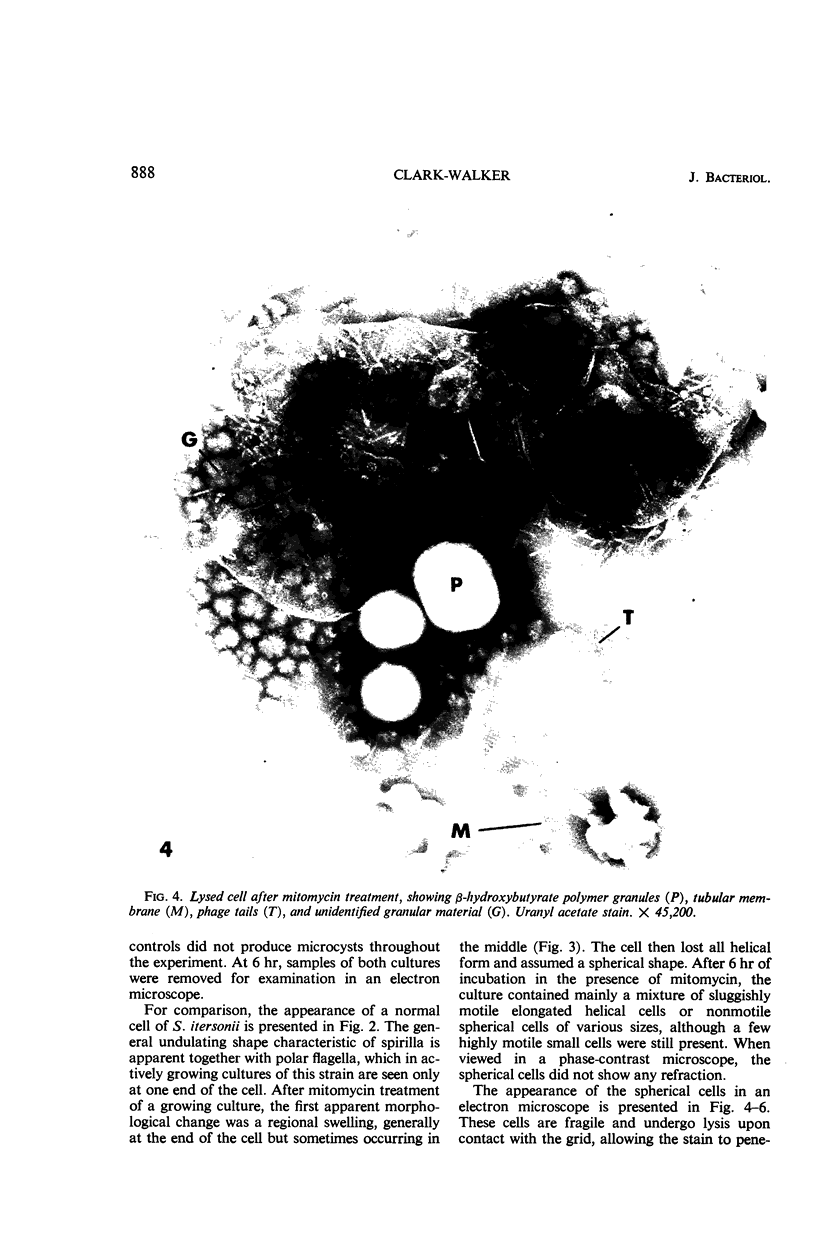
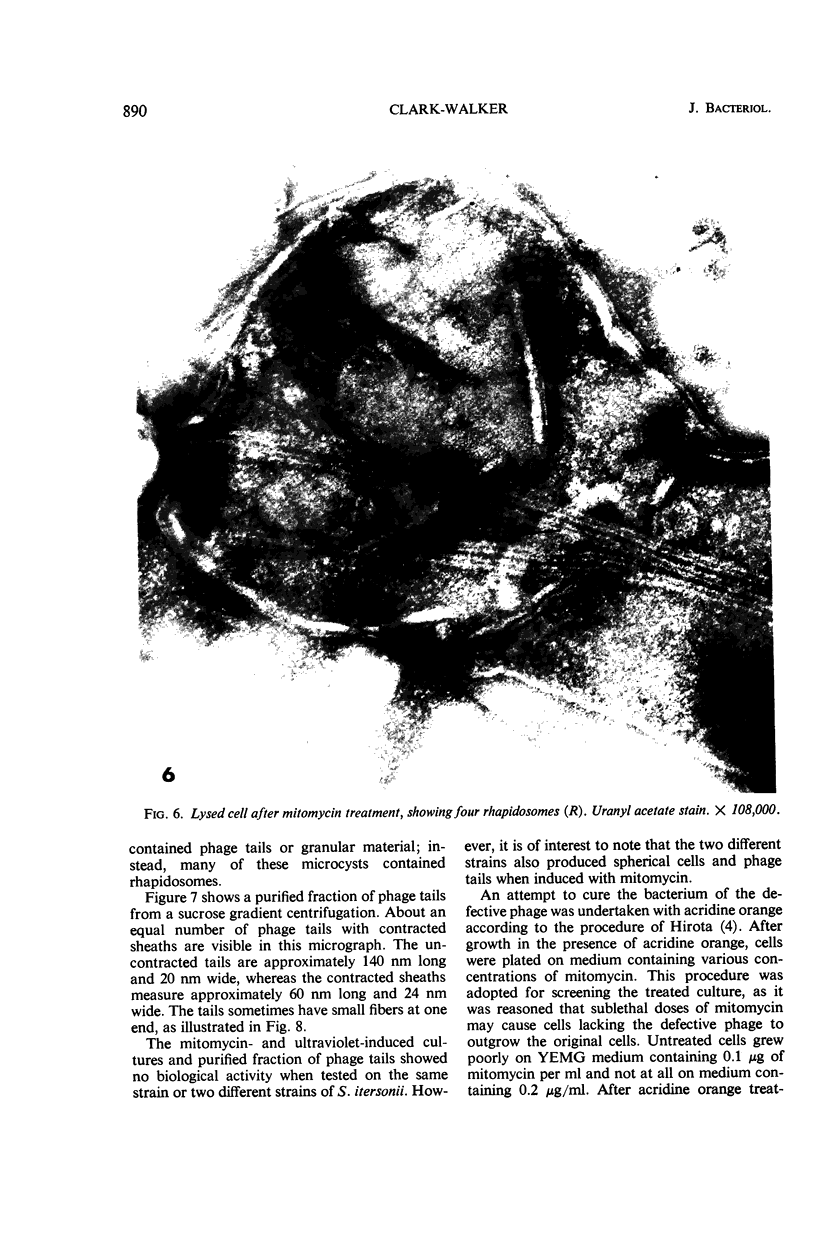
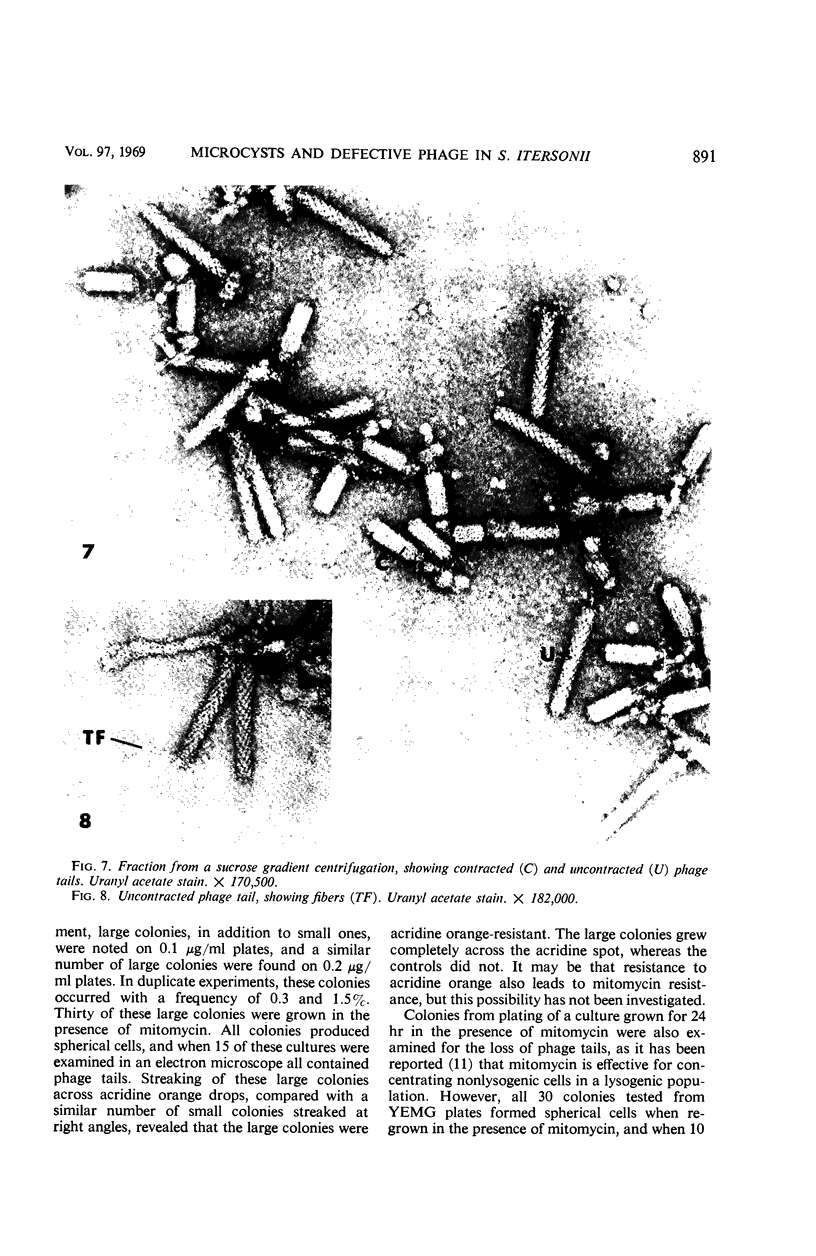
Mitomycin C and ultraviolet light were found to induce the formation of microcysts in Spirillum itersonii. These forms, as well as spontaneously occurring microcysts in this species, were found to contain phage tail parts, rhapidosomes, and a granular substance not seen in normal cells. It is suggested that microcysts are formed as the result of the induction of a defective phage. The production of phage lysozyme within the cell could lead to the formation of spherical forms as the cells lose their structural mucopeptide layer. Complete virus particles were not seen, nor was any biological activity demonstrated when the induced cultures were tested against two other strains of S. itersonii. The other strains of this bacterium also formed microcysts and phage tail parts when induced with mitomycin. Attempts to isolate an organism lacking the defective phage have been unsuccessful.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRELL D. L., LEWIN R. A. ROD-SHAPED RIBONUCLEOPROTEIN PARTICLES FROM SAPROSPIRA. Can J Microbiol. 1964 Feb;10:63–74. doi: 10.1139/m64-010. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., DELATOUR E. B. ON THE FINE STRUCTURE OF NORMAL AND "POLYMERIZED" TAIL SHEATH OF PHAGE T4. J Ultrastruct Res. 1964 Dec;11:545–563. doi: 10.1016/s0022-5320(64)80081-2. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- Pate J. L., Johnson J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. II. Structure and formation of rhapidosomes. J Cell Biol. 1967 Oct;35(1):15–35. doi: 10.1083/jcb.35.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., SHATKIN A. J., TATUM E. L. Bacteriocidal action of mitomycin C. Biochim Biophys Acta. 1961 Oct 14;53:132–149. doi: 10.1016/0006-3002(61)90800-9. [DOI] [PubMed] [Google Scholar]
- RITTENBERG S. C., WILLIAMS M. A. Microcyst formation and germination in Spirillum lunatum. J Gen Microbiol. 1956 Aug;15(1):205–209. doi: 10.1099/00221287-15-1-205. [DOI] [PubMed] [Google Scholar]
- Reichenbach H. Die wahre Natur der Myxobakterien- "Rhapidosomen". Arch Mikrobiol. 1967 Apr 17;56(4):371–383. [PubMed] [Google Scholar]
- Yamamoto T. Presence of rhapidosomes in various species of bacteria and their morphological characteristics. J Bacteriol. 1967 Nov;94(5):1746–1756. doi: 10.1128/jb.94.5.1746-1756.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]