Abstract
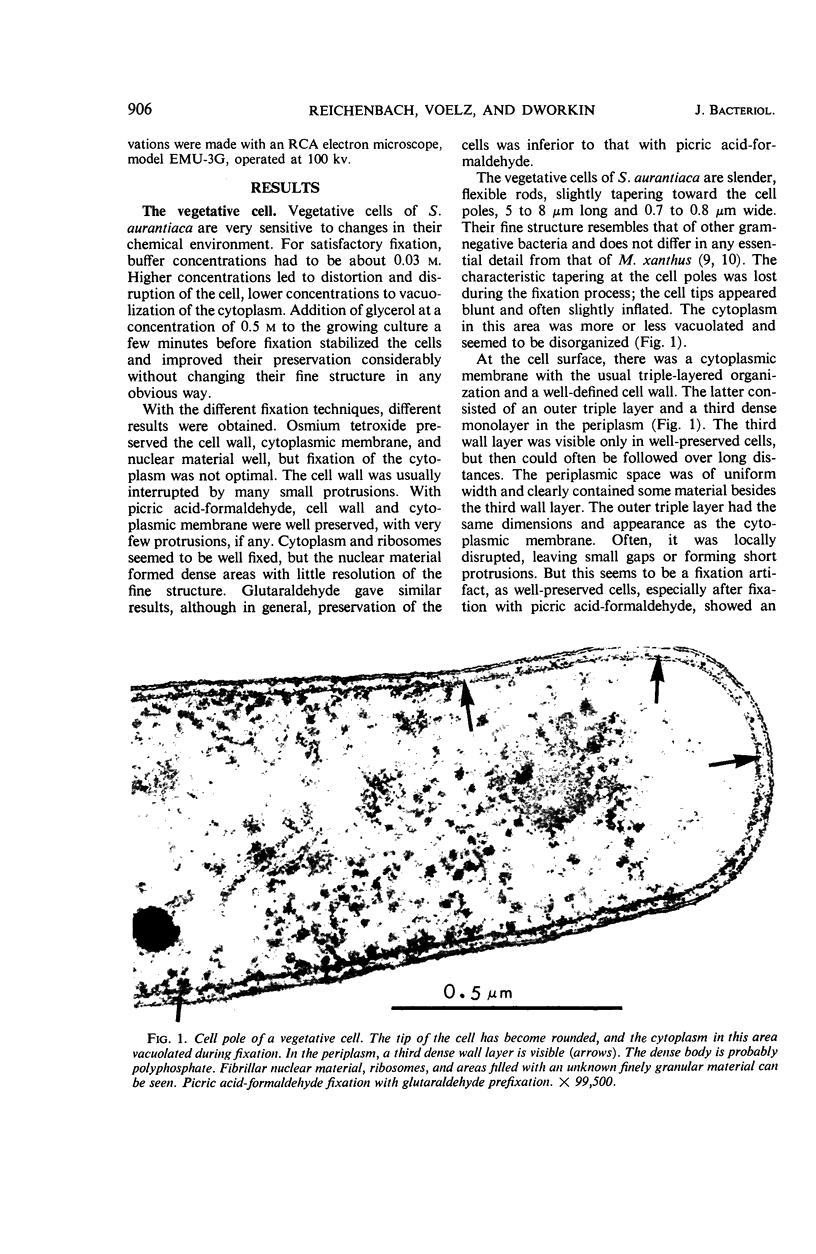
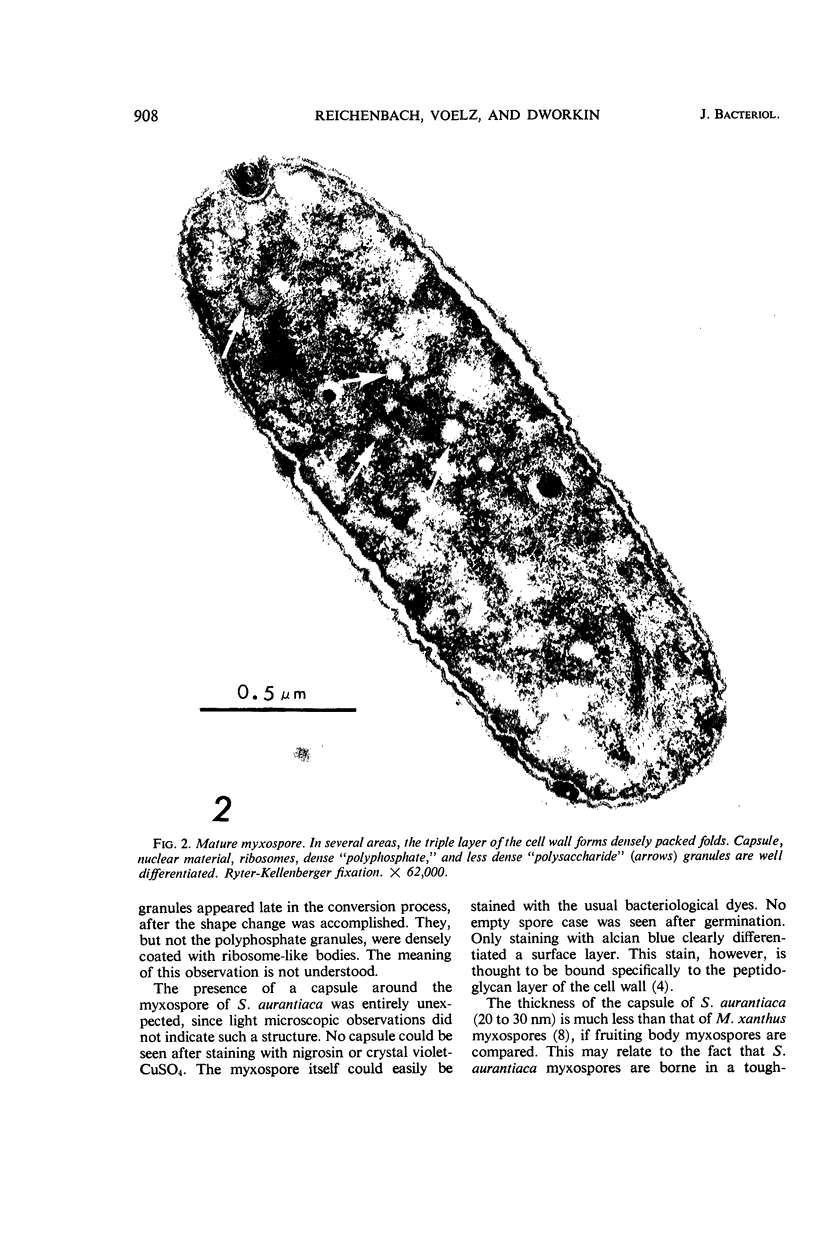
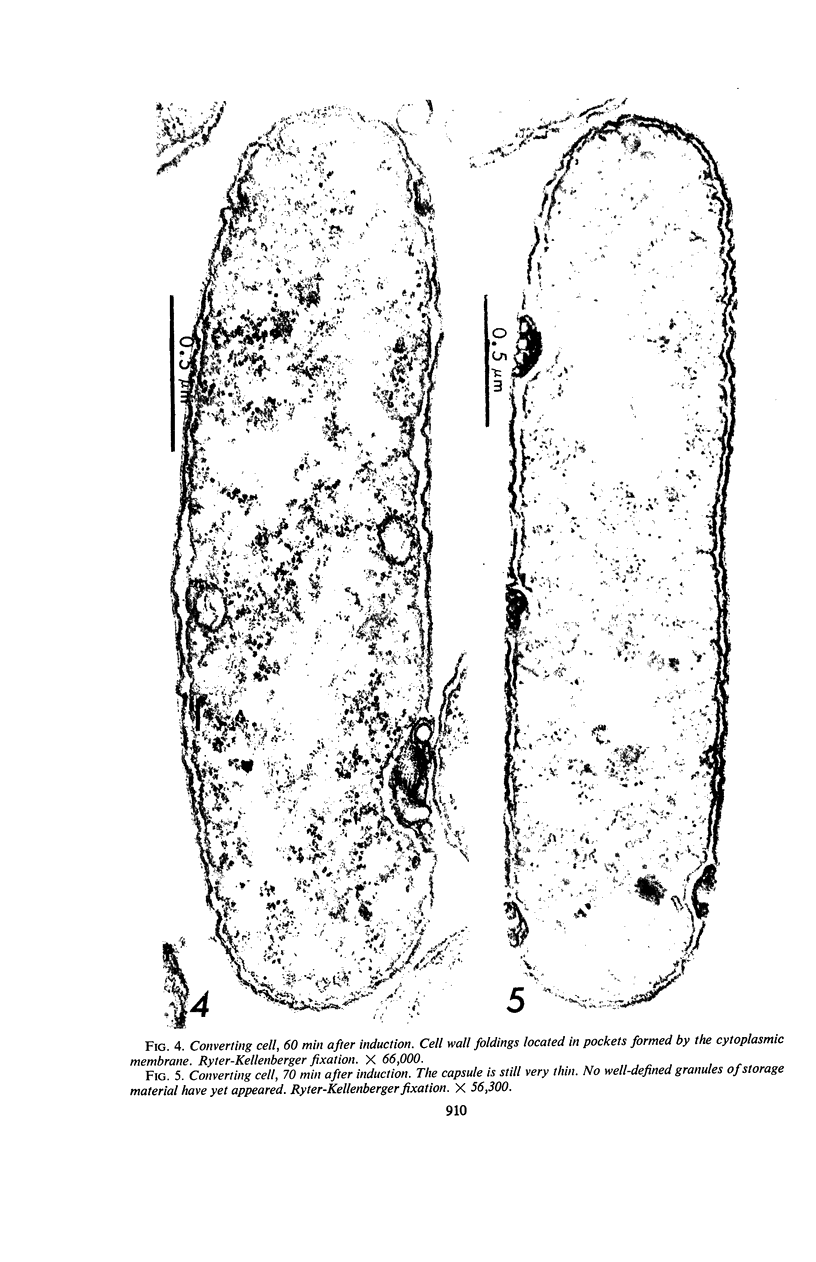
Suspension cultures of Stigmatella aurantiaca (Chondromyces aurantiacus) were induced to form myxospores by addition of glycerol to the growing culture. The cells were fixed at various stages during conversion, thin sections were prepared, and changes in fine structure were studied. Vegetative cells are quite similar in their ultrastructure to Myxococcus xanthus. During transformation into myxospores, three important cytological changes were observed. Granules of storage material, probably polysaccharide and polyphosphate, accumulated; a 200 to 300-μm thick capsule was laid down, and the outer triple layer of the cell wall became locally folded. These cell wall folds were often densely packed and lay in pockets formed by the cytoplasmic membrane. We have suggested the possibility that the cell may store in these folds wall material which has become superfluous by the decrease in surface area during conversion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- VOELZ H., DWORKIN M. Fine structure of Myxococcus xanthus during morphogenesis. J Bacteriol. 1962 Nov;84:943–952. doi: 10.1128/jb.84.5.943-952.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz H. The fate of the cell envelopes of Myxococcus xanthus during microcyst germination. Arch Mikrobiol. 1966 Nov 11;55(2):110–115. doi: 10.1007/BF00418633. [DOI] [PubMed] [Google Scholar]
- Voelz H. The physical organization of the cytoplasm in Myxococcus zanthus and the fine structure of its components. Arch Mikrobiol. 1967 Jun 6;57(2):181–195. doi: 10.1007/BF00408700. [DOI] [PubMed] [Google Scholar]
- Voelz H., Voelz U., Ortigoza R. O. The "polyphosphate overplus" phenomenon in Myxococcus xanthus and its influence on the architecture of the cell. Arch Mikrobiol. 1966 May 9;53(4):371–388. doi: 10.1007/BF00409874. [DOI] [PubMed] [Google Scholar]