Abstract
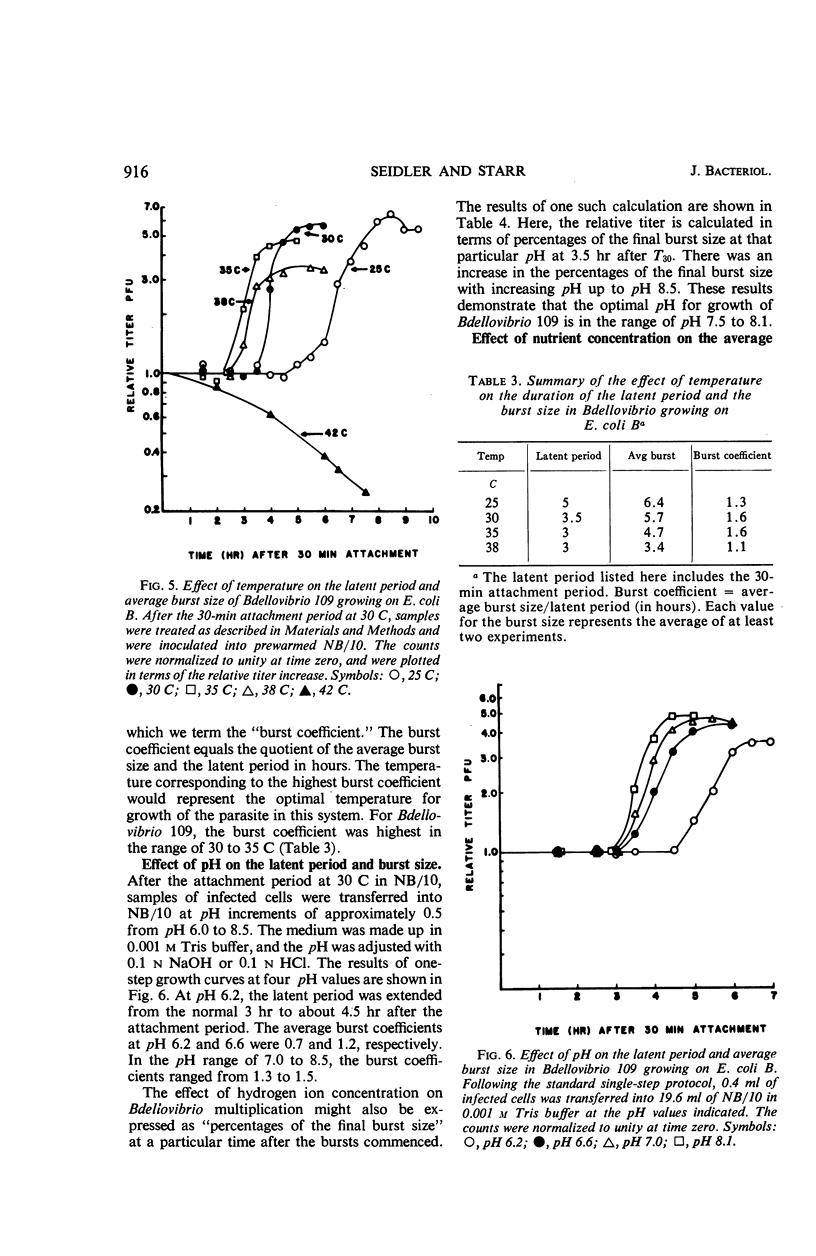
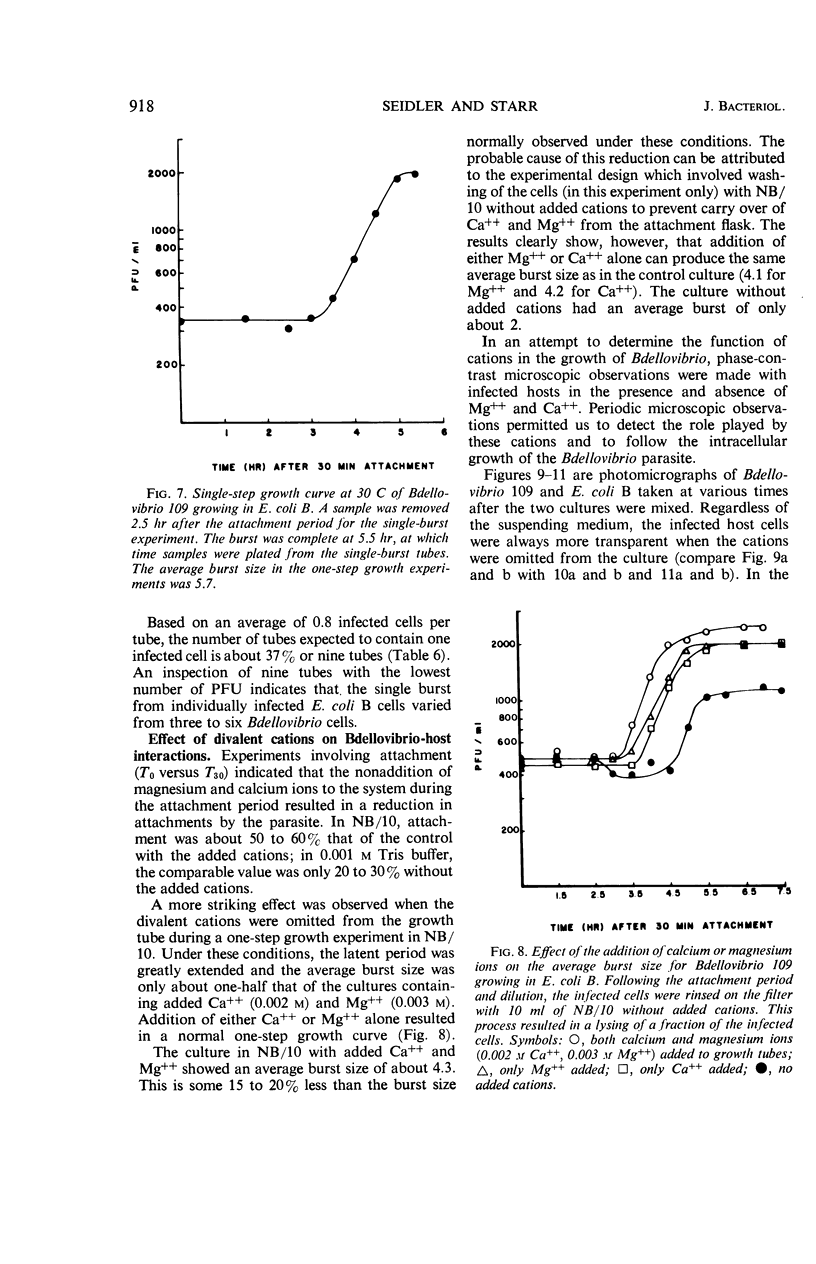
A procedure for one-step growth experiments on Bdellovibrio bacteriovorus growing parasitically in Escherichia coli B was developed. The resulting one-step growth curves showed that, under defined conditions at 30 C, each singly infected E. coli host cell, on the average, gave rise to 5.7 Bdellovibrio cells. This value was confirmed by single-burst experiments and by microscopic observations. In the temperature range of 25 to 38 C, the average burst size and the duration of the latent period were inversely proportional to the temperature. The effect of hydrogen ion concentration on the one-step growth kinetics in this system indicated a broad pH optimum, ranging from neutrality to slightly alkaline pH values. After Bdellovibrio cells and host cells were mixed, there was always a delay (the so-called “lag phase”) before the parasite titer increased in terms of plaque-forming units. Phase-contrast microscopic observations indicated that this delay stems in part from the polyphasic nature of the Bdellovibrio life cycle. We propose the following five terms to make explicit the sequence of events in this life cycle: “attachment,” “penetration,” “elongation,” “fragmentation,” and “burst.” Nutritional experiments revealed that Bdellovibrio obtains a major fraction of its cellular components from host-cell material. Infection of E. coli by Bdellovibrio without added Mg++ or Ca++ (0.003 m Mg++, 0.002 m Ca++) resulted in partial or total lysis of the host cell soon after infection. Protoplast integrity was necessary for the normal completion of the intracellular growth phase of Bdellovibrio in E. coli; normal development of the parasite took place only in the presence of Mg++ or Ca++.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LEDERBERG J., ST CLAIR J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lépine P., Guélin A., Sisman J., Lamblin D. Etude au microscope électronique de la lyse de Salmonella par Bdellovibrio bacteriovorus. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jun 19;264(25):2957–2960. [PubMed] [Google Scholar]
- Marr A. G., Harvey R. J., Trentini W. C. Growth and division of Escherichia coli. J Bacteriol. 1966 Jun;91(6):2388–2389. doi: 10.1128/jb.91.6.2388-2389.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Structure of the flagellum of Bdellovibrio bacteriovorus. J Bacteriol. 1968 May;95(5):1952–1955. doi: 10.1128/jb.95.5.1952-1955.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Baigent N. L. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J Bacteriol. 1966 May;91(5):2006–2017. doi: 10.1128/jb.91.5.2006-2017.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The nature of the ghosts obtained by lysozyme lysis of Bacillus megaterium. Exp Cell Res. 1956 Feb;10(1):214–221. doi: 10.1016/0014-4827(56)90087-8. [DOI] [PubMed] [Google Scholar]