Abstract
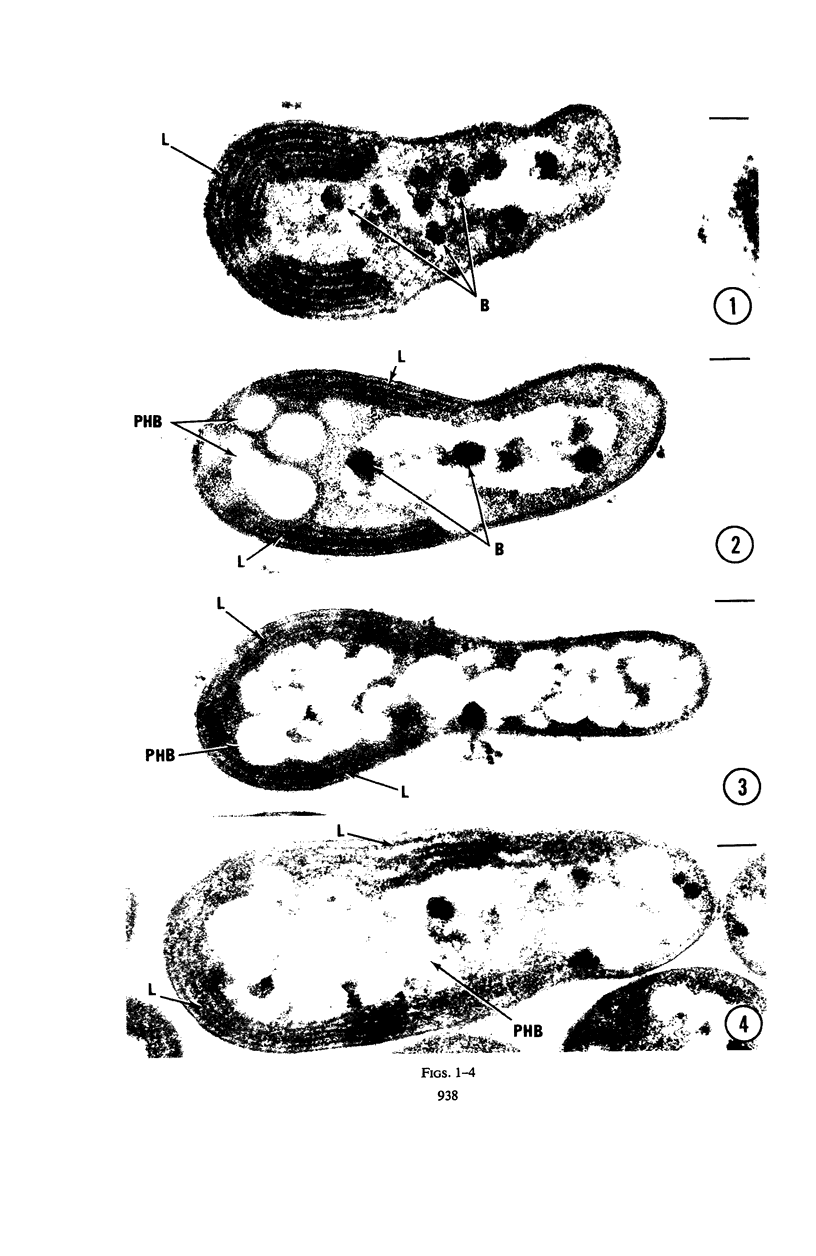
Nitrobacter agilis, grown through seven transfers heterotrophically in the absence of nitrite, was examined in the electron microscope. The ultrastructure of such cells closely resembled that of autotrophically grown N. agilis. It was thus futher established that the organisms growing heterotrophically were indeed N. agilis and, therefore, that N. agilis is a facultative autotroph. Acetate incorporation into poly-β-hydroxybutyrate was confirmed cytologically.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUGAN P. R., LUNDGREN D. G. ENERGY SUPPLY FOR THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. J Bacteriol. 1965 Mar;89:825–834. doi: 10.1128/jb.89.3.825-834.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Finstein M. S. Carbon and Energy Sources for the Nitrifying Autotroph Nitrobacter. J Bacteriol. 1965 Jul;90(1):102–107. doi: 10.1128/jb.90.1.102-107.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. Permanganate fixation of plant cells. J Biophys Biochem Cytol. 1959 Dec;6:431–436. doi: 10.1083/jcb.6.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney R. P., Edwards M. R. Fine Structure of Thiobacillus thiooxidans. J Bacteriol. 1966 Aug;92(2):487–495. doi: 10.1128/jb.92.2.487-495.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri S. M., Hoare D. S. Formic hydrogenlyase and the photoassimilation of formate by a strain of Rhodopseudomonas palustris. J Bacteriol. 1968 Jun;95(6):2344–2357. doi: 10.1128/jb.95.6.2344-2357.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P., Skerman V. B. Bacterial diversity: the natural history of selected morphologically unusual bacteria. Annu Rev Microbiol. 1965;19:407–454. doi: 10.1146/annurev.mi.19.100165.002203. [DOI] [PubMed] [Google Scholar]
- TOBBACK P., LAUDELOUT H. POLY-BETA-HYDROXYBUTYRIC ACID IN NITROBACTER. Biochim Biophys Acta. 1965 Mar 8;97:589–590. doi: 10.1016/0304-4165(65)90173-x. [DOI] [PubMed] [Google Scholar]
- Tauschel H. D., Drews G. Thylakoidmorphogenese bei Rhodopseudomonas palustirs. Arch Mikrobiol. 1967;59(4):381–404. [PubMed] [Google Scholar]
- Tilton R. C., Stewart G. J., Jones G. E. Marine Thiobacilli. II. Culture and ultrastructure. Can J Microbiol. 1967 Nov;13(11):1529–1534. doi: 10.1139/m67-201. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., McLee A. G. Rhodopseudomonas palustris and Rh. viridis--photosynthetic budding bacteria. Arch Mikrobiol. 1967;59(1):324–334. doi: 10.1007/BF00406346. [DOI] [PubMed] [Google Scholar]
- ZAVARZIN G., LEGUNKOVA R. The morphology of Nitrobacter winogradskyi. J Gen Microbiol. 1959 Aug;21:186–190. doi: 10.1099/00221287-21-1-186. [DOI] [PubMed] [Google Scholar]