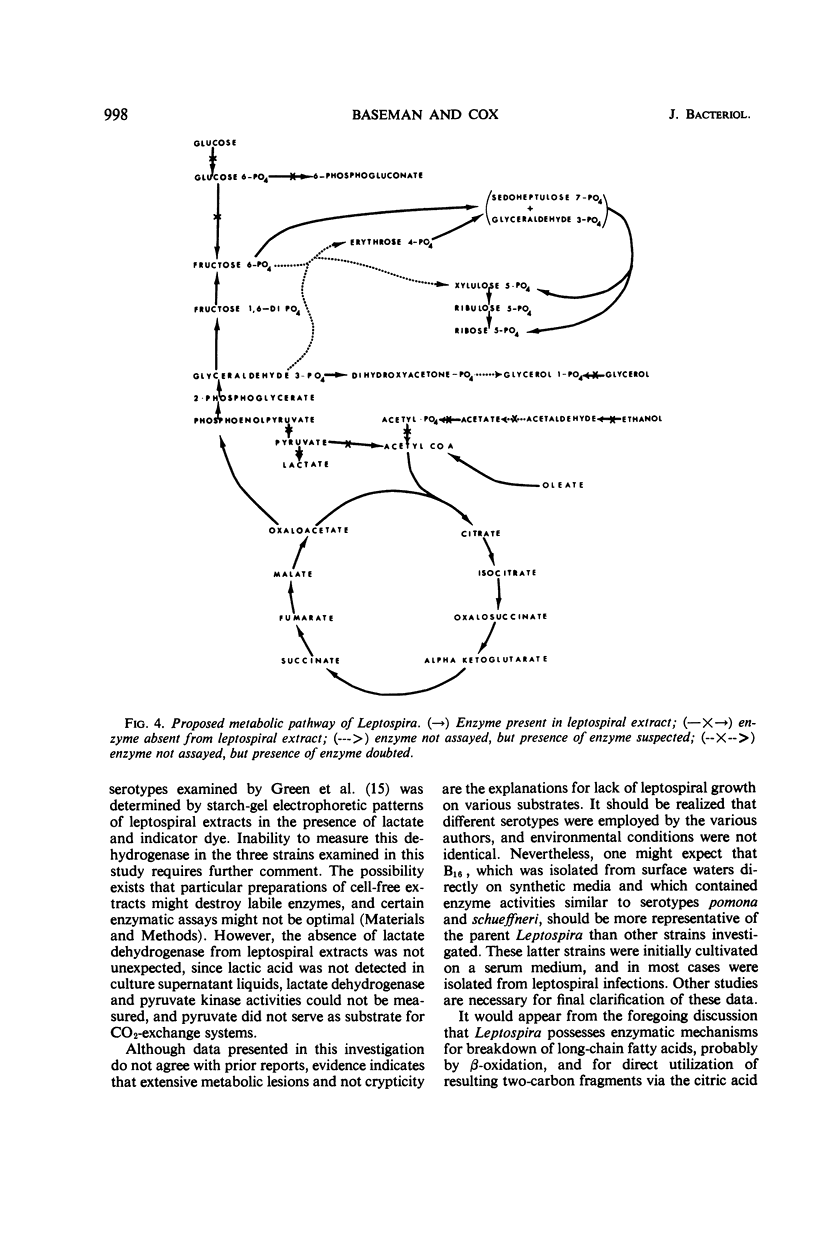
Abstract
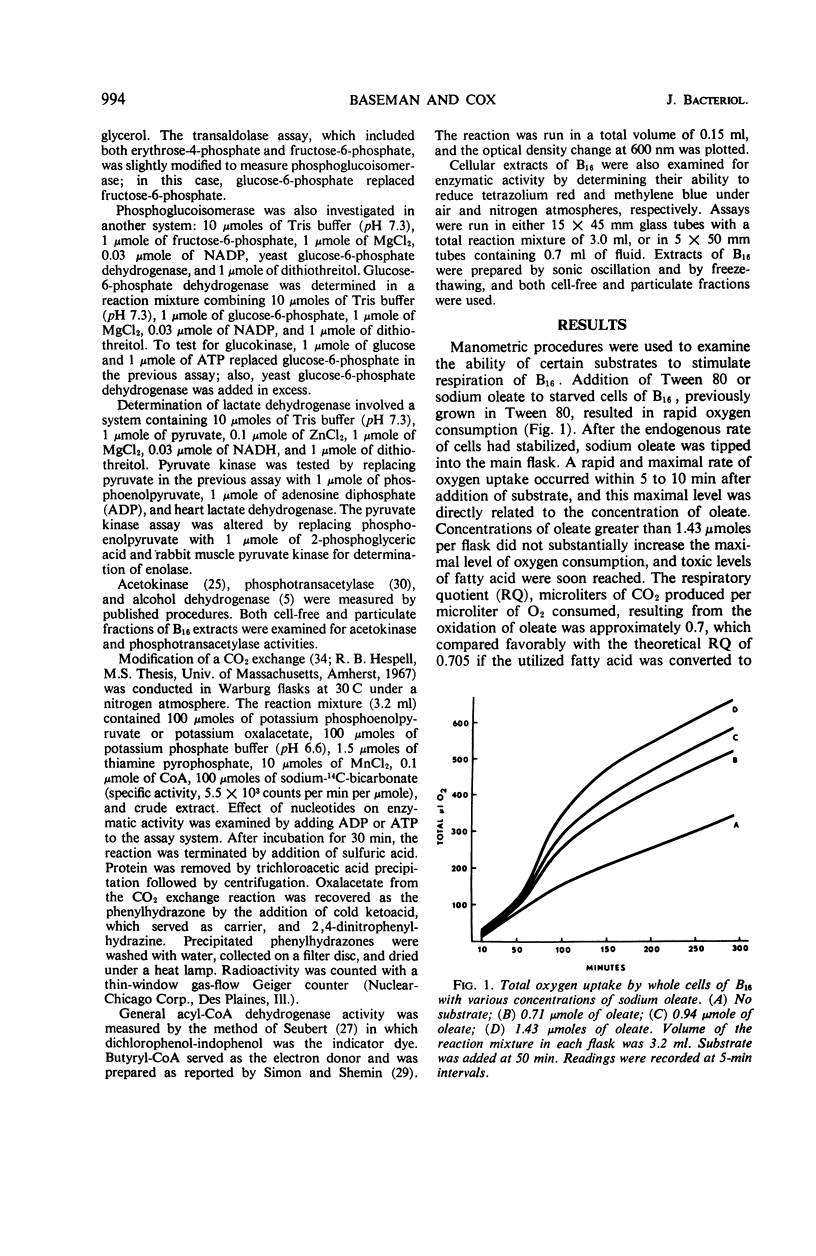
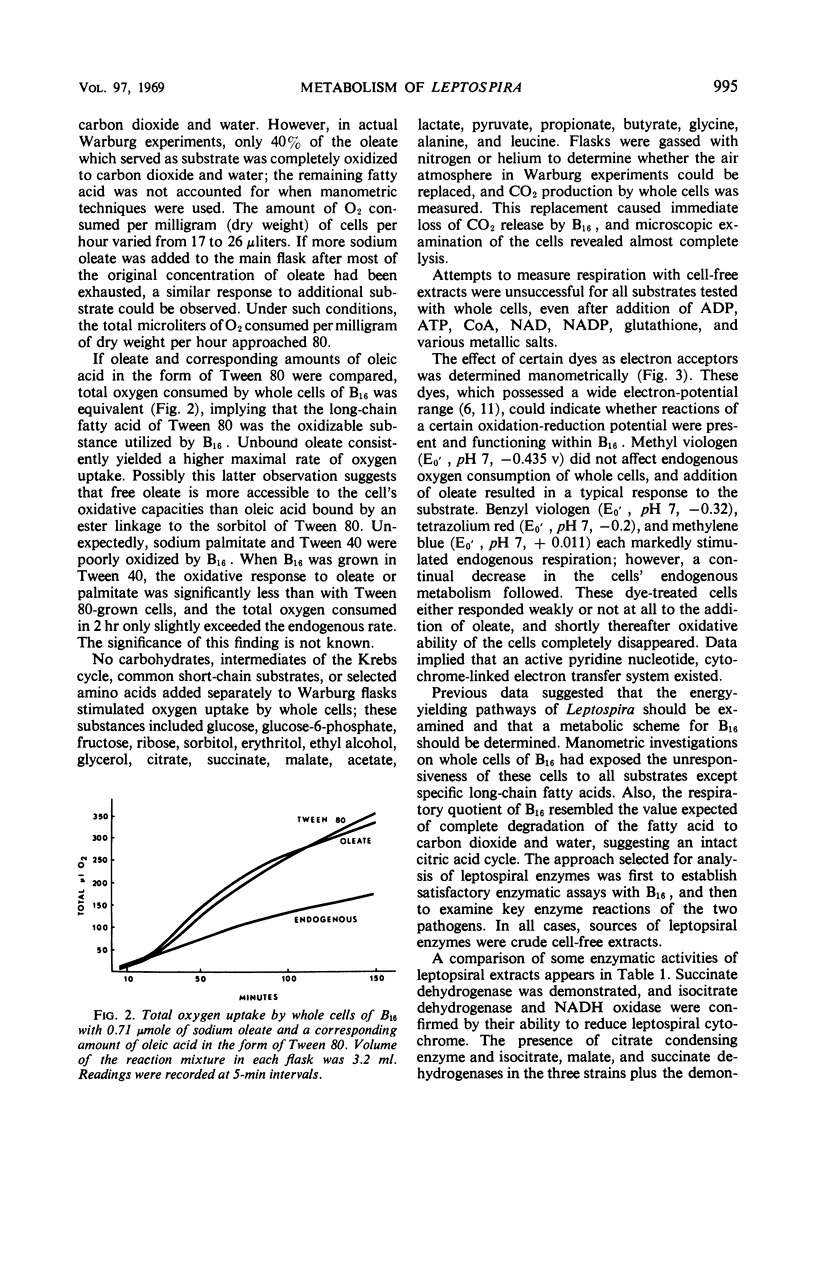
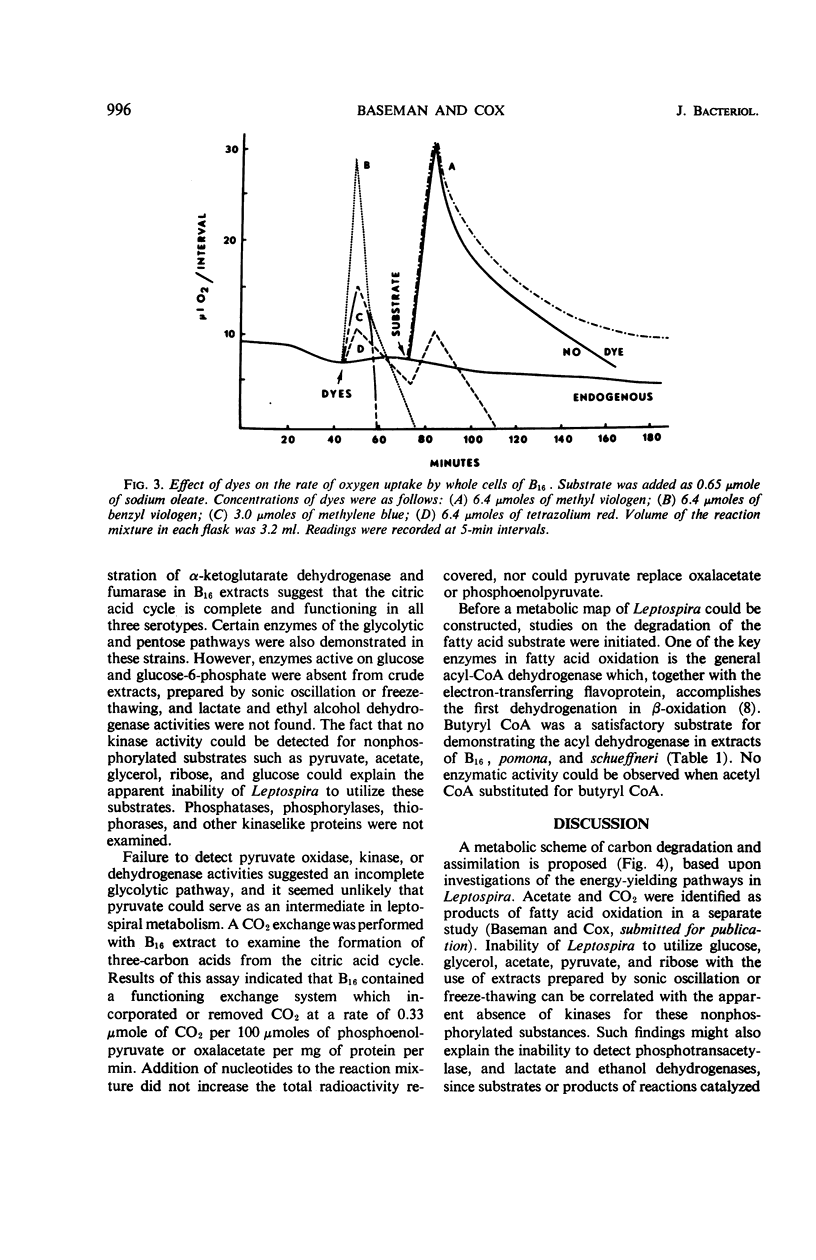
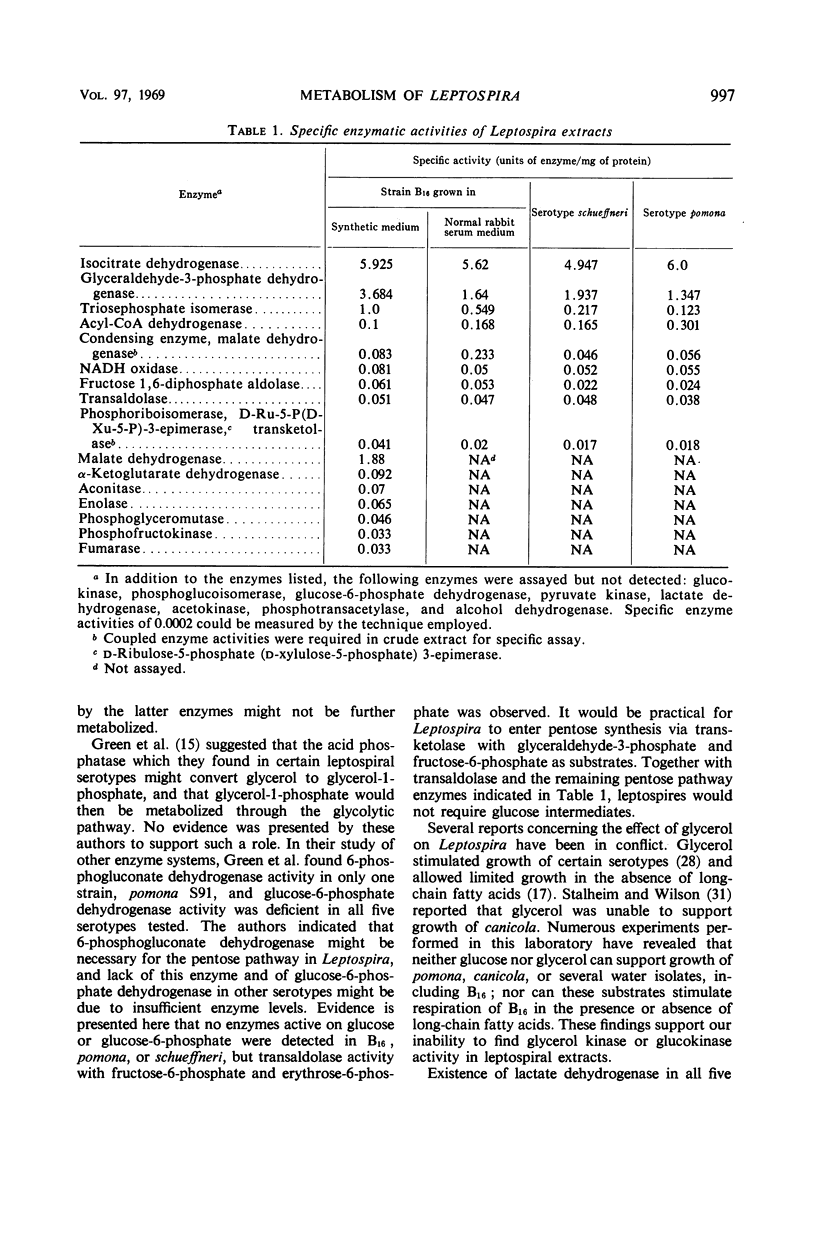
Metabolic studies were performed on three representative serotypes of Leptospira: a water isolate designated B16 and two pathogenic serotypes, pomona and schueffneri. Examination of whole cells of B16 for their ability to oxidize various substrates revealed that oleate significantly stimulated oxygen uptake. The respiratory quotient of 0.7 implied that oleate was degraded to carbon dioxide and water. Other substrates, such as carbohydrates, alcohols, intermediates of the citric acid cycle, and short-chain acids, including selected amino acids, did not stimulate endogenous respiration of whole cells. No oxygen uptake could be measured when cell-free extracts were tested with the substrates used with whole cells. Enzymatic analyses of cell-free extracts of the three strains demonstrated enzymes of the citric acid cycle, enzymes of the glycolytic and pentose pathways, and the general acyl coenzyme A dehydrogenase required for β-oxidation of fatty acids. Strain B16 and the two pathogenic serotypes appeared to possess similar metabolic capabilities. Enzymatic data might also explain the apparent inability of B16 to oxidize other substrates; kinases necessary for activation of common nonphosphorylated compounds were not detected in leptospiral extracts. These findings emphasized the dependence of leptospiral growth upon long-chain fatty acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Cox C. D. Terminal electron transport in Leptospira. J Bacteriol. 1969 Mar;97(3):1001–1004. doi: 10.1128/jb.97.3.1001-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Henneberry R. C., Cox C. D. Isolation and growth of Leptospira on artificial media. J Bacteriol. 1966 Mar;91(3):1374–1375. doi: 10.1128/jb.91.3.1374-1375.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX C. D., LARSON A. D. Colonial growth of leptospirae. J Bacteriol. 1957 Apr;73(4):587–589. doi: 10.1128/jb.73.4.587-589.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE F. L., MII S., HAUGE J. G., GREEN D. E., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. I. The general fatty acyl coenzyme A dehydrogenase. J Biol Chem. 1956 Feb;218(2):701–706. [PubMed] [Google Scholar]
- FAINE S. Catalase activity in pathogenic Leptospira. J Gen Microbiol. 1960 Feb;22:1–9. doi: 10.1099/00221287-22-1-1. [DOI] [PubMed] [Google Scholar]
- FULTON J. D., SMITH P. J. Carbohydrate metabolism in Spirochaeta recurrentis. 1. The metabolism of spirochaetes in vivo and in vitro. Biochem J. 1960 Sep;76:491–499. doi: 10.1042/bj0760491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON J. D., SPOONER D. F. The metabolism of leptospira icterohaemorrhagiae in vitro. Exp Parasitol. 1956 Mar;5(2):154–177. doi: 10.1016/0014-4894(56)90012-1. [DOI] [PubMed] [Google Scholar]
- GOLDBERG H. S., ARMSTRONG J. C. Oxidase reaction with leptospiral colonies and its adaptation to antibiotic sensitivity testing. J Bacteriol. 1959 Apr;77(4):512–513. doi: 10.1128/jb.77.4.512-513.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. S., Goldberg H. S., Blenden D. C. Enzyme patterns in the study of leptospira. Appl Microbiol. 1967 Sep;15(5):1104–1113. doi: 10.1128/am.15.5.1104-1113.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELPRIN J. J., HIATT C. W. The effect of fatty acids on the respiration of Leptospira icterohemorrhagiae. J Infect Dis. 1957 Mar-Apr;100(2):136–140. doi: 10.1093/infdis/100.2.136. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. C., GARY N. D. NUTRITION OF LEPTOSPIRA POMONA. II. FATTY ACID REQUIREMENTS. J Bacteriol. 1963 May;85:976–982. doi: 10.1128/jb.85.5.976-982.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky M. I., Hampp E. G. Metabolic gas production by a variety of spirochetes. J Dent Res. 1966 Jan-Feb;45(1):165–168. doi: 10.1177/00220345660450011001. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKOVETZ A. J., LARSON A. D. Transamination in Leptospira biflexa. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:638–640. doi: 10.3181/00379727-101-25044. [DOI] [PubMed] [Google Scholar]
- PATEL V., GOLDBERG H. S., BLENDEN D. CHARACTERIZATION OF LEPTOSPIRAL LIPASE. J Bacteriol. 1964 Oct;88:877–884. doi: 10.1128/jb.88.4.877-884.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO P. J., LARSON A. D., COX C. D. CATALASE ACTIVITY IN LEPTOSPIRA. J Bacteriol. 1964 Oct;88:1045–1048. doi: 10.1128/jb.88.4.1045-1048.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL C. M. A hemolysin associated with leptospirae. J Immunol. 1956 Dec;77(6):405–409. [PubMed] [Google Scholar]
- STALHEIM O. H., WILSON J. B. CULTIVATION OF LEPTOSPIRAE. I. NUTRITION OF LEPTOSPIRA CANICOLA. J Bacteriol. 1964 Jul;88:48–54. doi: 10.1128/jb.88.1.48-54.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenberg E. Growth of pathogenic Leptospira in chemically defined media. J Bacteriol. 1967 May;93(5):1598–1606. doi: 10.1128/jb.93.5.1598-1606.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELDKAMP H. Isolation and characteristics of Treponema zuelzerae nov. spec., and anaerobic, free-living spirochete. Antonie Van Leeuwenhoek. 1960;26:103–125. doi: 10.1007/BF02538999. [DOI] [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955 Aug;215(2):637–643. [PubMed] [Google Scholar]



