Abstract
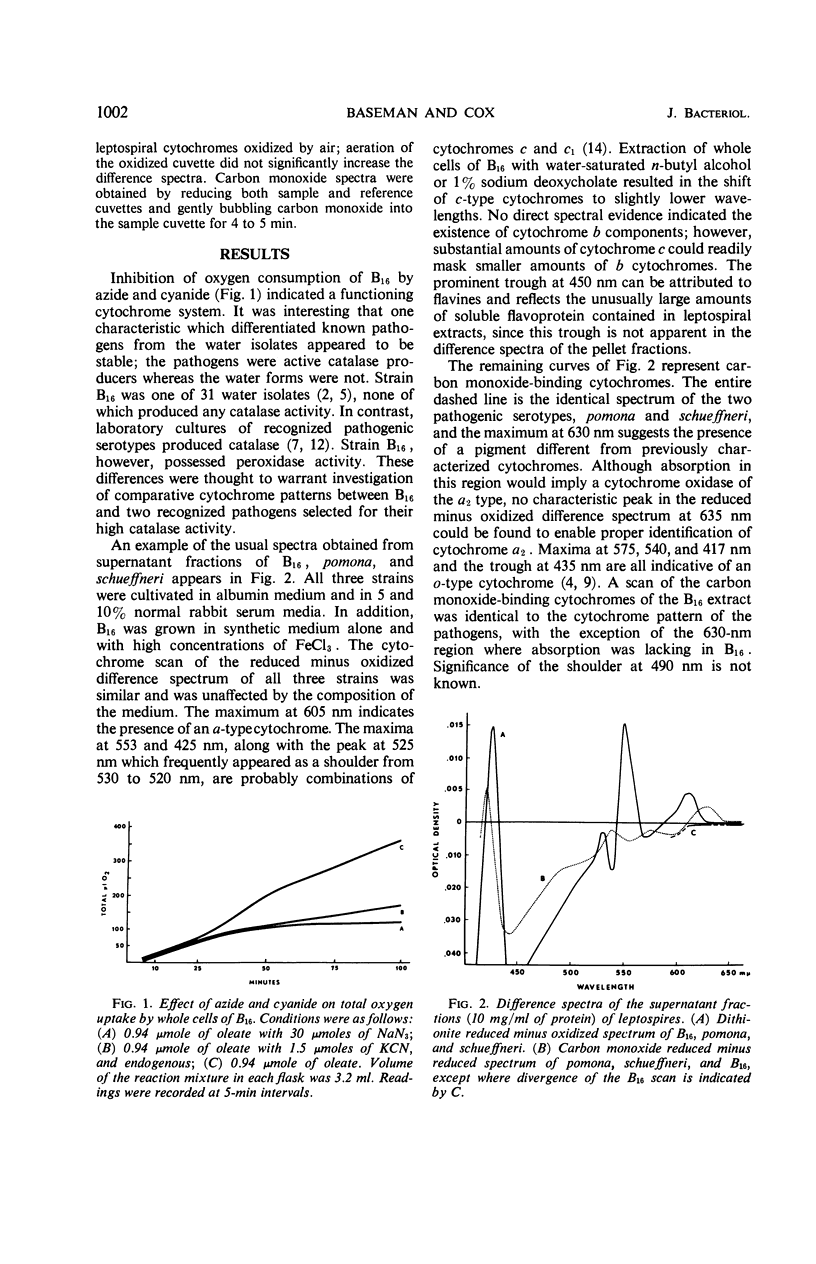
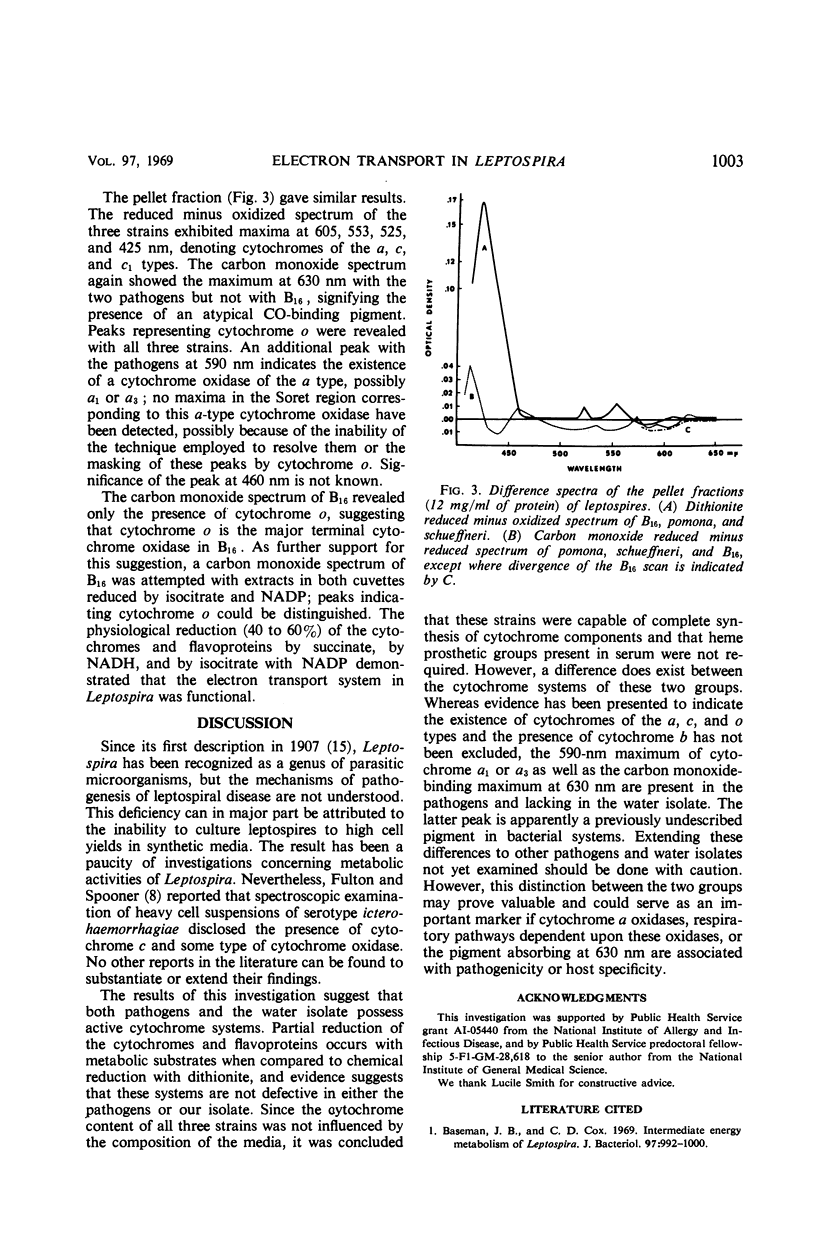
The cytochrome content of three leptospiral strains grown in several media was investigated after it was shown that respiratory inhibitors suppressed oxygen consumption of a water isolate, B16, and that two pathogenic serotypes, pomona and schueffneri, were active catalase producers, whereas B16 lacked catalase activity. Reduced minus oxidized difference spectra disclosed cytochromes of the a, c, and c1 types in all strains. Although no spectral evidence suggested the existence of cytochrome b components, they could have been masked by cytochrome c, and their presence cannot be ruled out. Carbon monoxide difference spectra revealed peaks indicative of a cytochrome oxidase of the o type in all strains. Carbon monoxide spectra further suggested that a cytochrome a oxidase, possibly a1 or a3, and a pigment with absorption spectra different from those of previously characterized cytochromes existed in the two pathogens and not in the water isolate. Physiological reduction of the cytochromes by various metabolic substrates implied that the cytochrome system in Leptospira was functional. No effect of the various growth media on the cytochrome patterns of the three strains was observed, indicating that all three strains were capable of synthesis of cytochrome components and did not require heme prosthetic groups present in serum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Cox C. D. Intermediate energy metabolism of Leptospira. J Bacteriol. 1969 Mar;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Henneberry R. C., Cox C. D. Isolation and growth of Leptospira on artificial media. J Bacteriol. 1966 Mar;91(3):1374–1375. doi: 10.1128/jb.91.3.1374-1375.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX C. D., LARSON A. D. Colonial growth of leptospirae. J Bacteriol. 1957 Apr;73(4):587–589. doi: 10.1128/jb.73.4.587-589.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Studies on the isolation and growth of Leptospira from surface waters. Ann Soc Belges Med Trop Parasitol Mycol. 1966;46(2):193–202. [PubMed] [Google Scholar]
- FAINE S. Catalase activity in pathogenic Leptospira. J Gen Microbiol. 1960 Feb;22:1–9. doi: 10.1099/00221287-22-1-1. [DOI] [PubMed] [Google Scholar]
- JACOBS N. J., CONTI S. F. EFFECT OF HEMIN ON THE FORMATION OF THE CYTOCHROME SYSTEM OF ANAEROBICALLY GROWN STAPHYLOCOCCUS EPIDERMIDIS. J Bacteriol. 1965 Mar;89:675–679. doi: 10.1128/jb.89.3.675-679.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RAO P. J., LARSON A. D., COX C. D. CATALASE ACTIVITY IN LEPTOSPIRA. J Bacteriol. 1964 Oct;88:1045–1048. doi: 10.1128/jb.88.4.1045-1048.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



