Abstract
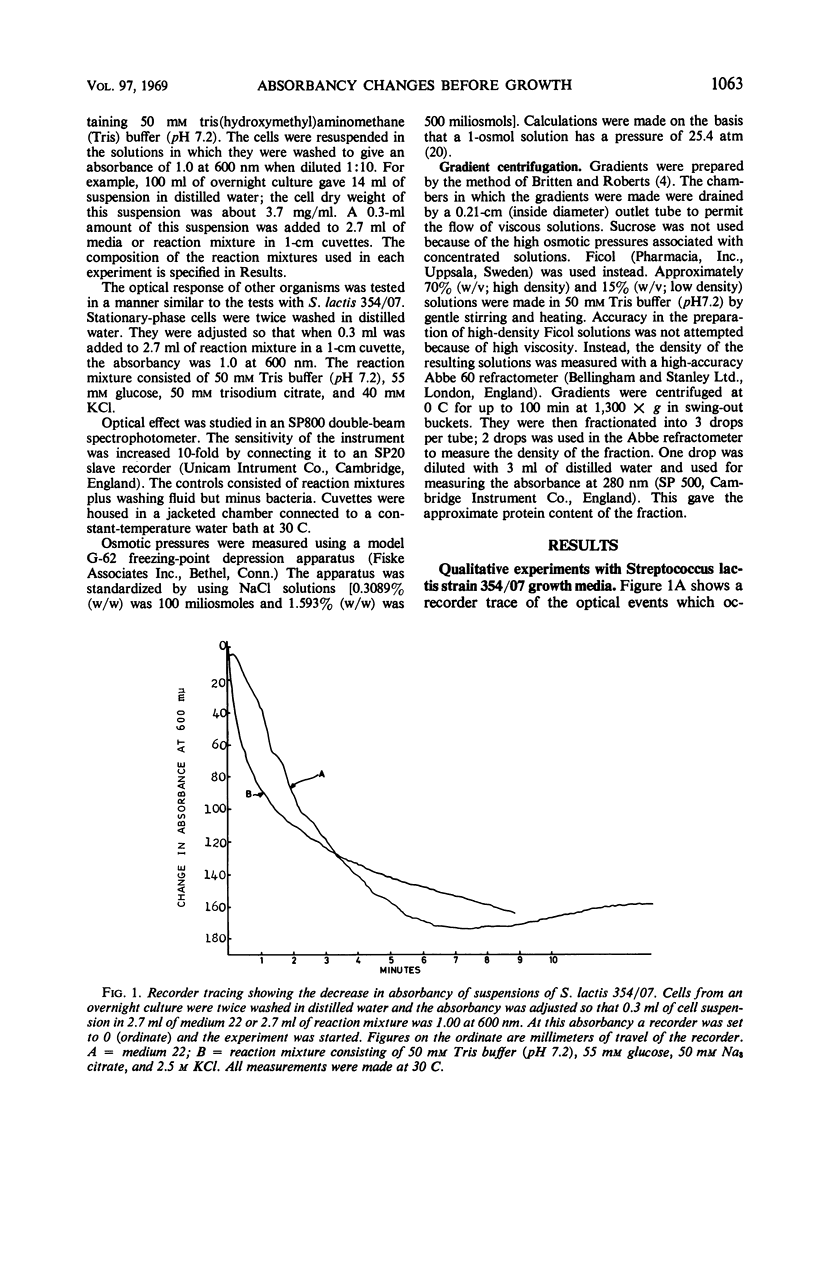
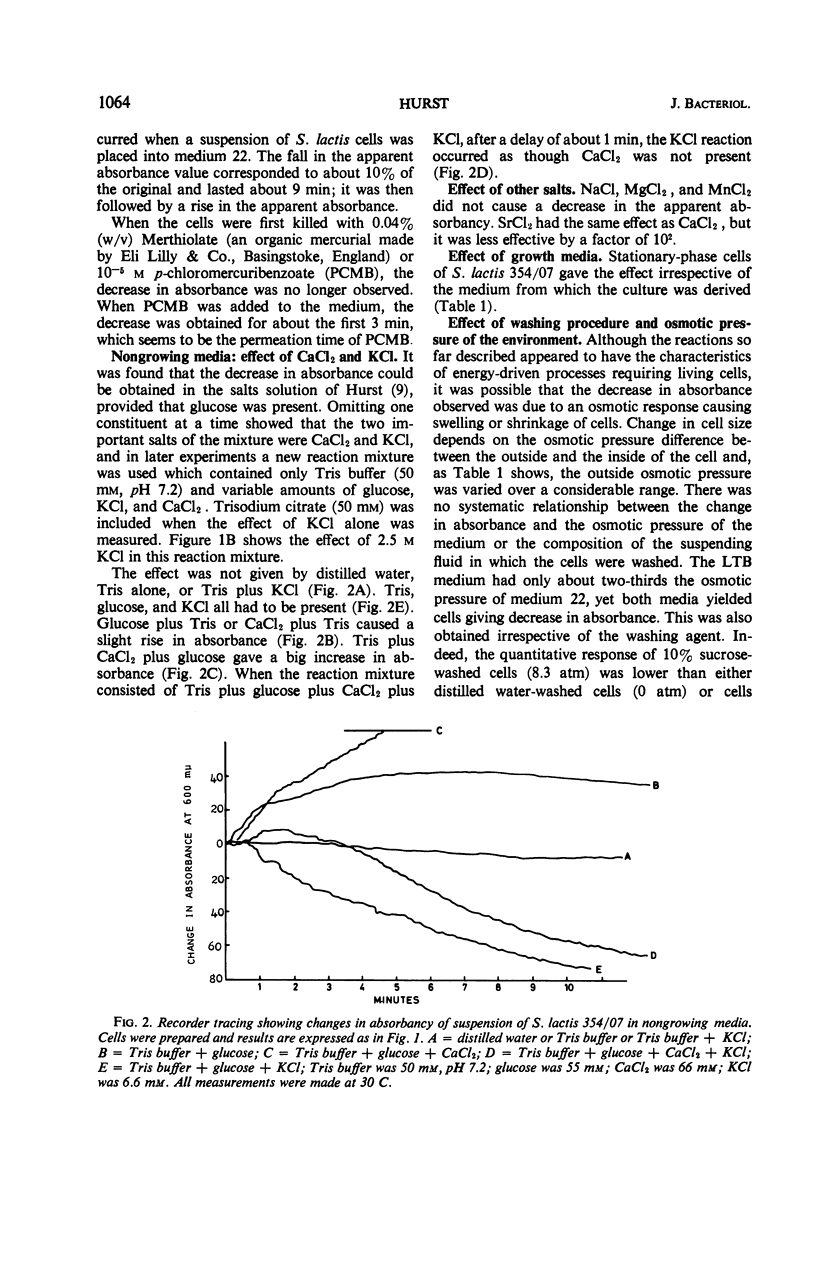
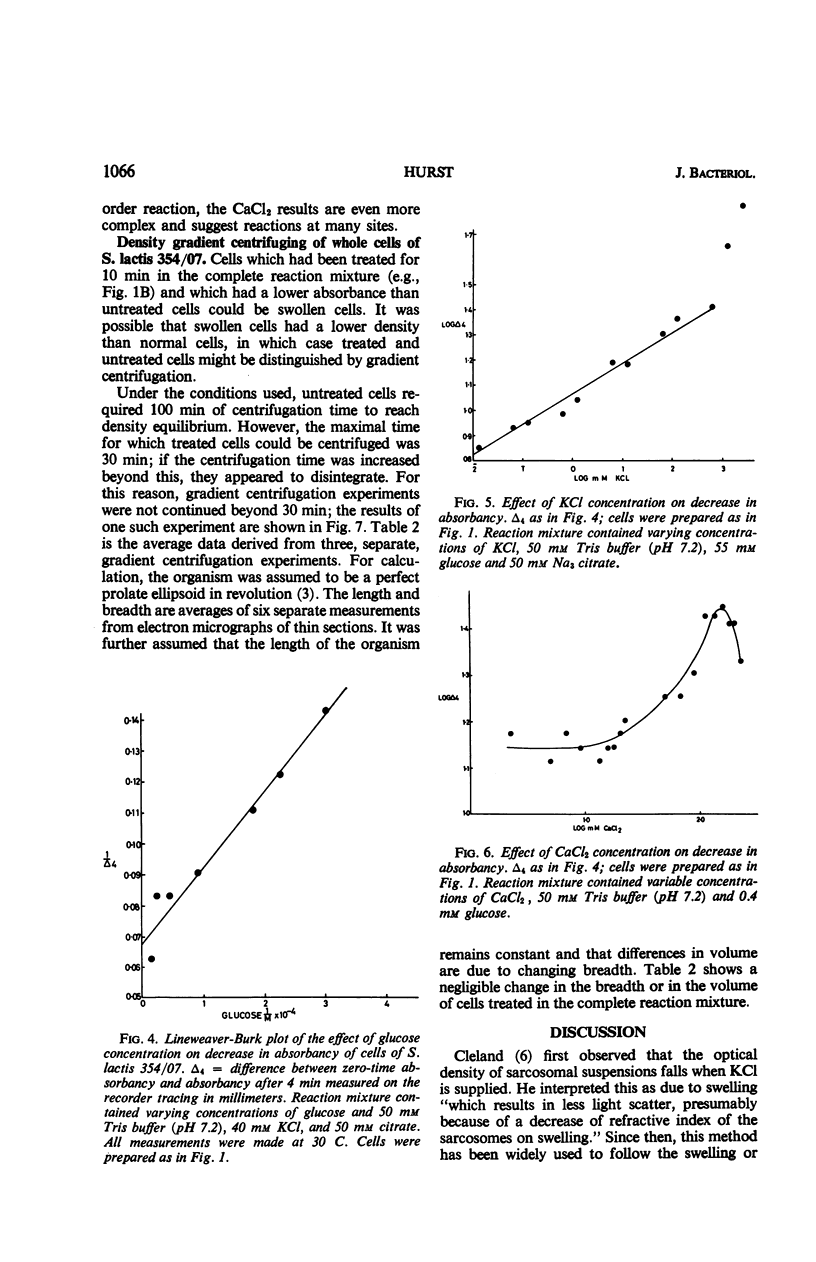
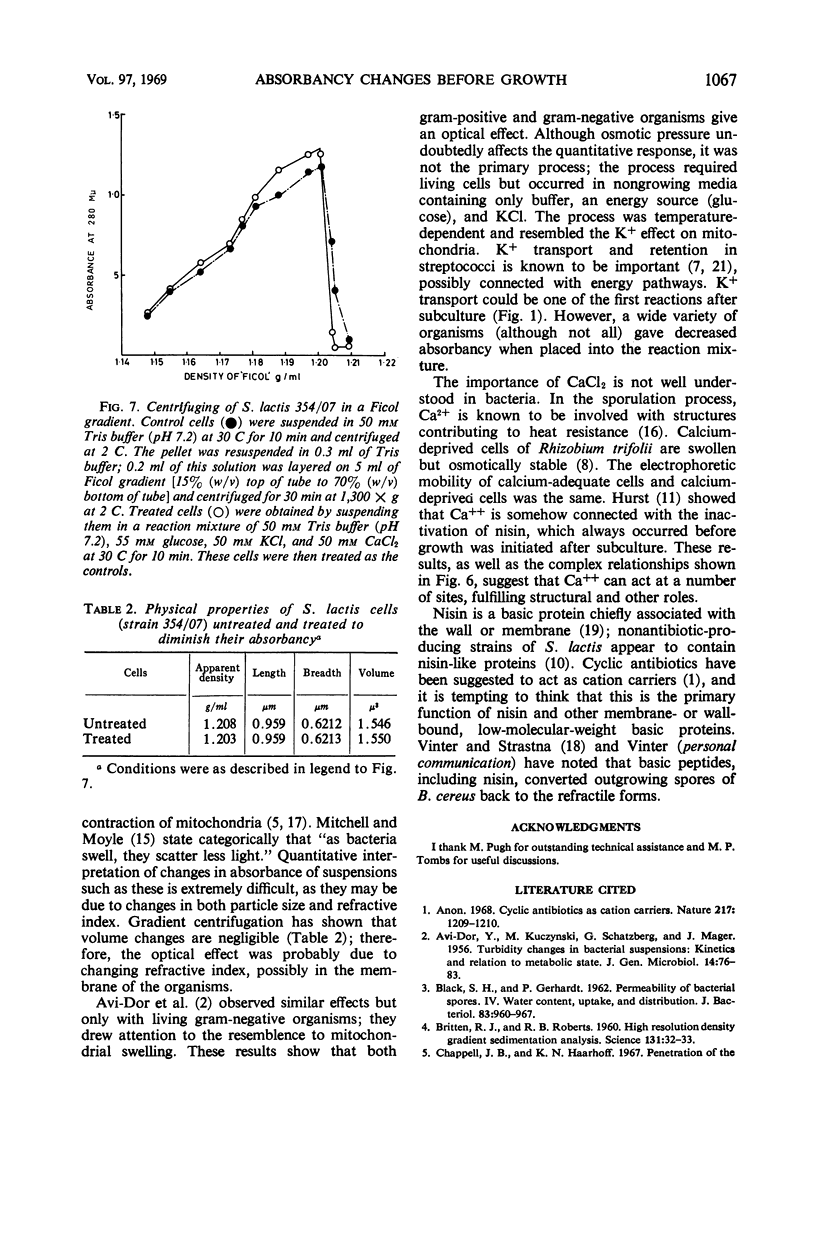
The apparent absorbancy of suspensions of stationary-phase cells of Streptococcus lactis strain 354/07 decreased immediately after being placed in fresh media. This optical effect also occurred in defined mixtures of buffer glucose and KCl. CaCl2 caused the absorbancy to increase. CaCl2 and KCl together had about the same effect as KCl alone. SrCl2 could replace CaCl2, but it was less effective by a factor of 102. MnCl2, MgCl2, and NaCl were without effect. The absorbancy did not change when cells were first killed by p-chloromercuribenzoate or when the reaction was carried out at 0 C. The rate of the reaction was dependent on temperature and concentration of glucose and salts. Gradient centrifugation suggests that this optical effect was caused by change in the refractive index of the test organism rather than by change in volume. Nine other organisms representing four additional genera gave the same optical effect as S. lactis 354/07. Two other organisms reacted feebly whereas another strain of S. lactis reacted in the opposite way, the absorbancy of the suspension increasing instead of decreasing. Spores of Bacillus cereus did not respond.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. IV. Water content, uptake, and distribution. J Bacteriol. 1962 May;83:960–967. doi: 10.1128/jb.83.5.960-967.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- CLELAND K. W. Permeability of isolated rat heart sarcosomes. Nature. 1952 Sep 20;170(4325):497–499. doi: 10.1038/170497a0. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of potassium transport by sodium in a mutant of Streptococcus faecalis. Biochemistry. 1967 Oct;6(10):3107–3110. doi: 10.1021/bi00862a018. [DOI] [PubMed] [Google Scholar]
- Humphrey B. A., Marshall K. C., Vincent J. M. Electrophoretic mobility of calcium-adequate and calcium-deprived Rhizobium trifolii. J Bacteriol. 1968 Feb;95(2):721–721. doi: 10.1128/jb.95.2.721-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Apparent destruction of nisin by the producer organism before initiation of growth in Streptococcus lactis. Nature. 1968 Jul 27;219(5152):403–404. doi: 10.1038/219403b0. [DOI] [PubMed] [Google Scholar]
- Hurst A. Biosynthesis of the antibiotic nisin by whole Streptococcus lactus organisms. J Gen Microbiol. 1966 Aug;44(2):209–220. doi: 10.1099/00221287-44-2-209. [DOI] [PubMed] [Google Scholar]
- Hurst A. Function of nisin and nisin-like basic proteins in the growth cycle of streptococcus lactis. Nature. 1967 Jun 17;214(5094):1232–1234. doi: 10.1038/2141232a0. [DOI] [PubMed] [Google Scholar]
- Hurst A., Lazarus W. Calcium uptake during growth of Streptococcus lactis. Nature. 1968 Jul 27;219(5152):404–405. doi: 10.1038/219404a0. [DOI] [PubMed] [Google Scholar]
- MAGER J., KUCZYNSKI M., SCHATZBERG G., AVI-DOR Y. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J Gen Microbiol. 1956 Feb;14(1):69–75. doi: 10.1099/00221287-14-1-69. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter V., Stastná J. Spores of microorganisms. XXI. Conversion of outgrowing spores of Bacillus cereus to refractile forms by basic peptides and proteins. Folia Microbiol (Praha) 1967;12(3):301–307. doi: 10.1007/BF02868748. [DOI] [PubMed] [Google Scholar]
- White R. J., Hurst A. The location of nisin in the producer organism, Streptococcus lactis. J Gen Microbiol. 1968 Sep;53(2):171–179. doi: 10.1099/00221287-53-2-171. [DOI] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]


