Abstract
The biological activity of the transcription factor NF-κB is mainly controlled by the IκB proteins IκBα and IκBβ, which restrict NF-κB in the cytoplasm and enter the nucleus where they terminate NF-κB-dependent transcription. In this paper we describe the cloning and functional characterization of mouse IκBɛ. Mouse IκBɛ contains 6 ankyrin repeats required for its interaction with the Rel proteins and is expressed in different cell types where we found that it is up-regulated by NF-κB inducers, as is the case for IκBα and human IκBɛ. IκBɛ functions as a bona fide IκB protein by restricting Rel proteins in the cytoplasm and inhibiting their in vitro DNA binding activity. Surprisingly, IκBɛ did not inhibit transcription of genes regulated by the p50/p65 heterodimer efficiently, such as the human interferon-β gene. However, IκBɛ was a strong inhibitor of interleukin-8 expression, a gene known to be regulated by p65 homodimers. In addition, IκBɛ appears to function predominantly in the cytoplasm to sequester p65 homodimers, in contrast with the other two members of the family, IκBα and IκBβ, which also function in the nucleus to terminate NF-κB-dependent transcriptional activation.
The transcription factor NF-κB plays a major role in the activation of numerous genes involved in the function and development of the immune system, in the recruitment of leukocytes from the circulation into extravascular space, and in inflammatory and acute responses, etc. (reviewed in refs. 1–4). NF-κB consists of homo- and heterodimeric proteins that belong to the Rel family of transcription factors. In mammals there are five Rel proteins, p50, p52, p65 (RelA), c-Rel, and RelB, all of which contain a so-called Rel homology region (RHR) that includes DNA-binding and dimerization domains and a nuclear localization signal (NLS). The Rel proteins are present in most cell types in an inactive cytosolic form. The cellular partitioning of NF-κB is tightly regulated by the IκB proteins, which are complexed with NF-κB in the cytoplasm (5). The NF-κB DNA binding activity can be induced by a large variety of extracellular signals, all of which culminate in the proteolytic degradation of IκBs by the proteasome, thereby freeing NF-κB to translocate to the nucleus, where it activates gene transcription (1–7).
There are two known types of Rel complexes in the cytoplasm. The first type consists of heterodimers containing the p65 or c-Rel proteins associated with the precursors for p50 or p52 (p105 and p100, respectively). The second type consists of p65 and c-Rel homo- or heterodimers (with p50 or p52) associated with a member of the IκB family. A common characteristic of the IκB proteins is the presence of multiple copies of a motif, the ankyrin repeat, which interacts with the RHR. This interaction has two functional consequences. First, NF-κB–IκB complexes are sequestered in the cytoplasm, because the IκBs mask the NLS through direct protein–protein interactions; and second, IκBs can prevent NF-κB from binding to the DNA in vitro and in vivo. The IκB family consists of IκBα (5 ankyrin repeats) (8), IκBβ (6 ankyrin repeats) (9), IκBɛ (6 ankyrin repeats) (10), and the precursors p105 and p100, each bearing 7 ankyrin repeats at their carboxyl termini (reviewed in refs. 1–7). The BCL3 nuclear protein, containing 6 ankyrin repeats, can either coactivate transcription through p50 and p52 homodimers or inhibit their DNA binding (11, 12).
Despite their structural similarities the IκB proteins IκBα and IκBβ appear to play different roles in vivo. For example, the rapid and transient activation of NF-κB in response to tumor necrosis factor-α (TNF-α) is because of the degradation of IκBα alone, with no effect on IκBβ (9). The transient response is because of the fact that NF-κB activates transcription of the IκBα gene resulting in the de novo synthesis of IκBα protein (13). The newly synthesized IκBα resets the switch in the cytoplasm and enters the nucleus to terminate NF-κB-dependent transcription (14). In contrast, the persistent activation of NF-κB by inducers such as interleukin 1 (IL-1), lipopolysaccharide, or Tax I is because of the degradation of both IκBα and IκBβ, and the activity of NF-κB persists for several hours despite the de novo synthesis of IκBα protein (9). The reason for this is that newly synthesized IκBβ, which is unphosphorylated, is part of the NF-κB activating complex in the nucleus, and this ternary complex is refractory to IκBα inhibition (14, 15). Thus, the IκBα and IκBβ containing Rel complexes respond to different inducers in vivo, and furthermore, IκBα and IκBβ have distinct properties in regulating NF-κB DNA binding in the nucleus. Unlike IκBα and IκBβ, IκBɛ is associated in the cytoplasm exclusively with p65 homodimers and/or p65/c-Rel heterodimers and not with p50 or p52 containing heterodimers (10). However, the role of IκBɛ in postinduction repression of NF-κB as well as the significance of its exclusive association with p65 or c-Rel has not been addressed. On cellular activation, IκBɛ protein is degraded with slow kinetics by the proteasome-dependent pathway (10).
In this paper we describe the cloning and functional characterization of the mouse IκBɛ gene. We show that despite the fact that IκBɛ shares structural and some functional similarities with IκBα and IκBβ, it differs on the mechanisms by which it inhibits the activity of NF-κB. We show that IκBɛ functions mainly in the cytoplasm to sequester specifically the p65 homodimeric form of NF-κB, whereas IκBα and IκBβ share the additional function of entering the nucleus to inhibit DNA binding of p65 containing homo- or heterodimeric forms of NF-κB.
MATERIALS AND METHODS
The Yeast Two-Hybrid Screening.
The yeast two-hybrid selection and screen protocol were performed essentially as previously described (CLONTECH technical manual). The DNA encoding the RHR of p65 (amino acids 1–325) was cloned in frame with the GAL4 DNA-binding domain in the vector pY2 (16). The Saccharomyces cerevisiae strain HF7C (CLONTECH) harboring the pY2-p65RHR plasmid was transformed with a cDNA library fused to the GAL4 activation domain prepared from poly(A)+ RNA isolated from phorbol 12-myristate 13-acetate (PMA)-induced WEHI-3 cells (obtained from S. Goff, Columbia University), with the lithium acetate method as previously described (17). HF7C bears the HIS3 and lacZ genes under the control of GAL4 sites. Colonies that grew in media lacking histidine and turned blue were tested for plasmid linkage and for interaction with the GAL4 DNA-binding domain.
Plasmid Constructions.
Mammalian expression vectors for IκBα and IκBβ have been previously described. To construct the IκBɛ expression vector we cloned the full-length IκBɛ cDNA between the BamHI and XbaI sites in the pCDNA3 plasmid (Invitrogen). The GAL4-p65FL plasmid and the PRDII-chloramphenicol acetyltransferase (CAT) and −110IFN-β-CAT reporters have been previously described (14). The IL-8 luciferase reporter and an expression vector containing the VP16 activation domain–SV40NLS cassette were kindly provided by K. Leclair (Harvard Univ., Cambridge, MA) and I. Sadowski (Univ. of British Columbia, Vancouver), respectively.
Transfection, Cell Culture, and Northern Blotting.
P19, COS, or 3T3 cells were transfected by the calcium phosphate method as previously described. Transfections were carried out with the concentration of plasmids indicated in the figure legends, and in every case vector DNA was added as necessary to achieve a constant amount of transfected DNA. Virus, TNF-α, or PMA inductions were carried out as previously described (18).
Northern blots were carried out by using standard procedures (19). Briefly, 30 μg of total RNA per sample were analyzed in 1.2% agarose-formaldehyde gels, transferred to Hybond-N+ membranes and hybridized with a random primed 32P IκBɛ cDNA fragment.
Electrophoretic Mobility Shift Assays (EMSAs) and Expression of Proteins in Escherichia coli.
EMSAs using recombinant proteins or cell extracts were performed as previously described (14).
The expression and purification of NF-κB proteins IκBα and IκBβ have been previously described. To express IκBɛ and IκBɛΔC in E. coli, the corresponding regions were amplified by PCR and cloned into the PRSET expression vector (Invitrogen). Proteins were expressed and purified as previously described (14).
Whole, Nuclear, and Cytoplasmic Extract Preparation.
Whole cell extracts from transfected COS cells were prepared as follows. Sixty hours posttransfection the cells (60-mm dish) were washed 3 times in PBS, harvested by scraping, and pelleted at 14,000 rpm for 5 s in an Eppendorf microcentrifuge. The cell pellet was resuspended in 100 ml of extraction buffer containing 20 mM Hepes, pH 7.9, 300 mM KCl, 0.2 mM EDTA, 0.5% Nonidet P-40, 1 mM DTT, 7% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg/ml pepstatin A, and 10 μg/ml aprotinin. The cells were lysed by three rounds of freeze-thawing and spun at 14,000 rpm, and the supernatant was frozen in dry ice and stored at −80°C. Nuclear and cytoplasmic extracts were prepared as previously described (20). Western blots were carried out as previously described (14).
RESULTS
Cloning, Structure, and Expression of Mouse IκBɛ.
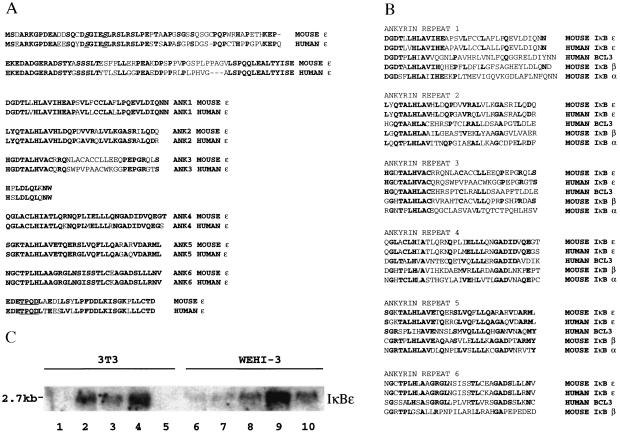
We used the yeast two-hybrid system to clone cDNAs whose products interact with the RHR of the p65 subunit of NF-κB. Plasmid DNA was isolated from all 178 primary positive yeast clones and used in a new round of transformation, where we found that 69 clones encoded proteins that specifically interact with the RHR of p65. The nucleotide sequence obtained from both ends of these clones revealed that 32 were IκBα, 20 were p105, 8 were p100, and 2 were IκBβ. Two of the remaining clones were identical and encoded an ORF that contained multiple ankyrin repeats with significant homology to the IκB proteins, whereas the rest of the clones encoded other novel proteins. The 2-ankyrin repeat-containing clones were named IκBɛ because they correspond to the mouse homologue of the recently described human IκBɛ protein (10). The full-length cDNA is 2,446 bp long and contains an ORF of 364 aa, whereas the human IκBɛ cDNA contains an ORF of 500 aa (10). However, the human protein is translated from an internal ATG codon (10) that corresponds to the mouse initiation codon. Fig. 1A shows the deduced 364-aa ORF of the mouse IκBɛ gene and its maximum alignment with the human homologue (80% identity). There are two regions of significant sequence divergence between the mouse and human proteins, at the amino terminus and in the middle of the third ankyrin repeat (Fig. 1A). Interestingly, the mouse IκBɛ sequence in the latter region is more similar to the other IκBs than the human IκBɛ is (Fig. 1B). The mouse IκBɛ protein contains 6 ankyrin repeats with homology to the other IκB proteins (Fig. 1B). Importantly, the similarity between ankyrin repeats in the same position of different IκBs is greater than the similarity between ankyrin repeats in the same molecule (Fig. 1B). In contrast to IκBα and IκBβ, mouse IκBɛ contains a long amino terminus of 121 aa and a short carboxyl terminus of 32 aa flanking the 6 ankyrin repeats. Similarly to IκBα and IκBβ, there are two serine residues at the amino terminus at positions 18 and 22, which in the human IκBɛ protein have been shown to be critical for its degradation in response to extracellular signals (10). Finally, the carboxyl terminus of IκBɛ, although it does not contain a typical PEST sequence like the other IκBs (21–23), is rich in acidic residues (9 acidic aa of 32), as are the termini of IκBα and IκBβ.
Figure 1.
Primary structure and expression of the mouse IκBɛ gene. (A) Shown is the deduced amino acid sequence of the mouse IκBɛ ORF aligned to the human homologue. Identical amino acids are in bold. The two serine residues corresponding to those found in the other IκBs are in underlined bold italics. The amino terminus, the 6 ankyrin repeats, and the carboxyl terminus are shown separately. The TPQD sequence shown in bold and underlined at the carboxyl terminus corresponds to the consensus casein kinase II site. (B) Shown is the maximum alignment of the ankyrin repeat sequences found in IκBɛ, IκBα, IκBβ, and BCL3. Identities are indicated in bold. (C) Expression of the mouse IκBɛ gene. Shown is a Northern blot containing total RNA isolated from mouse 3T3 (lanes 1–5) and WEHI-3 (lanes 6–10) cells hybridized with the IκBɛ cDNA. Lanes 1 and 6, uninduced cells; lanes 2 and 7, TNF-α induction for 4 h; lanes 3 and 8, TNF-α induction for 12 h; lanes 4 and 9, virus infection for 12 h; lanes 5 and 10, PMA induction for 12 h.
To examine whether mouse IκBɛ gene expression parallels NF-κB activation, as is the case for IκBα (13) and human IκBɛ (10), we performed Northern blot analysis with RNA isolated from mouse 3T3 fibroblasts and WEHI3 macrophages induced with several NF-κB inducers for various times. Fig. 1C shows that in 3T3 fibroblasts the basal level expression of the IκBɛ mRNA (2.7 kb) is almost undetectable (lane 1), but it is significantly increased following TNF-α treatment (lanes 2 and 3) or virus infection (lane 4). Interestingly, PMA treatment does not induce IκBɛ expression in 3T3 cells (lane 5), although the human IκBɛ gene is induced after PMA/ionomycin treatment of HL60 or Jurkat cells (10). In contrast, the basal level of expression is higher in WEHI3 cells (lane 6) and is further induced after treatment with TNF-α (lanes 7 and 8), virus infection (lane 9), or PMA (lane 10). These experiments taken together with the analysis of the human IκBɛ gene expression (10) suggest that NF-κB may up-regulate the expression of IκBɛ in a negative autoregulatory loop similar to IκBα.
IκBɛ Removes NF-κB from the DNA in Vitro.
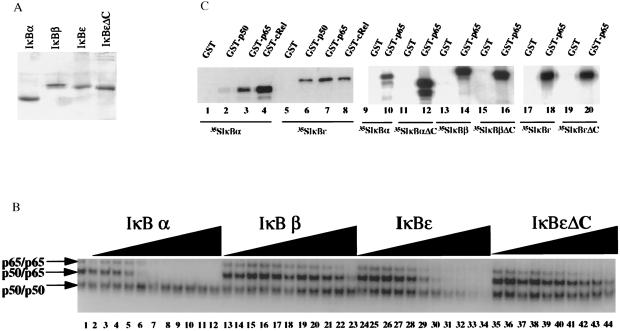
A common property shared by all members of the IκB family is their ability to inhibit NF-κB DNA binding (reviewed in ref. 7). To examine the ability of IκBɛ to remove NF-κB from the DNA, we performed EMSAs with recombinant NF-κB and IκB proteins. The full-length IκBɛ protein as well as a carboxyl-terminal deletion derivative lacking the acidic tail were expressed and purified to near homogeneity from bacteria as 6 histidine fusions. Fig. 2A shows an SDS/PAGE gel stained with Coomassie blue displaying the purified IκBɛ, IκBɛΔC, IκBα, and IκBβ proteins. A constant amount of an equimolar mixture of the p50/p65 heterodimer as well as the corresponding p50 and p65 homodimers were allowed to interact with the PRDII oligonucleotide (the NF-κB site taken from the interferon-β (IFN-β) promoter) and then challenged with increasing amounts of IκB proteins. Consistent with our previous experiments, IκBα removed NF-κB from the DNA 10–20 times more efficiently than IκBβ (Fig. 2B, compare lanes 2–12 with 13–23) (14). Significantly, IκBɛ also removed NF-κB efficiently from the DNA whereas IκBɛΔC was 20 times weaker as an inhibitor of NF-κB DNA binding (lanes 24–34 and 35–44, respectively). Quantitation of several similar in vitro experiments revealed that IκBɛ is only a 2- to 3-fold weaker inhibitor of NF-κB DNA binding compared with IκBα. These effects are specific to NF-κB complexes containing the p65 subunit because none of the IκBs significantly affected DNA binding by the p50 homodimers (Fig. 2B). Identical results were obtained when NF-κB and IκB proteins were preincubated followed by the addition of the probe (data not shown).
Figure 2.
IκBɛ removes NF-κB from the DNA in vitro. (A) Shown is a Coomassie blue-stained SDS/PAGE gel displaying the purified IκB proteins used in this study. (B) Shown is an EMSA experiment performed with an equimolar mixture of p50 and p65 homodimers and p50/p65 heterodimer in the presence or absence of increasing concentrations of recombinant IκBα, IκBβ, IκBɛ, and IκBɛΔC. The molar ratio between IκB proteins and NF-κB proteins was 1:16, 1:8, 1:4, 1:2, 1:1, 2:1, 3:1, 6:1, 10:1, 16:1, and 24:1. (C) Protein–protein interactions between members of the Rel and IκB family of proteins. Lanes 1–8, in vitro translated and 35S-labeled IκBα (lanes 1–4) and IκBɛ (lanes 5–8) were incubated with glutatione beads harboring GST alone (lanes 1 and 5), GST-p50 (lanes 2 and 6), GST-p65 (lanes 3 and 7), or GST-cRel (lanes 4 and 8). Lanes 9–20, in vitro translated and 35S-labeled IκBα (lanes 9 and 10), IκBαΔC (lanes 11 and 12), IκBβ (lanes 13 and 14), IκBβΔC (lanes 15 and 16), IκBɛ (lanes 17 and 18), and IκBɛΔC (lanes 19 and 20) were incubated with glutathione beads harboring GST alone (lanes 9, 11, 13, 15, 17, and 19) or GST-p65 (lanes 10, 12, 14, 16, 18, and 20). The bound proteins were analyzed by PAGE and visualized by autoradiography.
Fig. 2C shows that in vitro translated and 35S-labeled IκBα and IκBɛ are specifically retained on glutathione beads containing glutathione S-transferase (GST)-p65, GST-cRel, and GST-p50 but not on GST alone (lanes 1–8). Deletion of the carboxyl terminus in any of the IκBs did not affect their interaction with p65 (lanes 9–20). Thus, similarly to IκBα and IκBβ, IκBɛ can interact in solution with p50 homodimers, but this interaction does not result in inhibition of DNA binding. In addition, in vivo IκBɛ is found exclusively associated with p65 and c-Rel and not with other Rel proteins (10). Finally, as is the case for IκBα and IκBβ, although the carboxyl-terminal acidic tail of IκBɛ is required for efficient inhibition of NF-κB DNA binding, it is not necessary for interaction with the Rel proteins.
IκBɛ Preferentially Inhibits the p65 Homodimeric Form of NF-κB in Vivo.
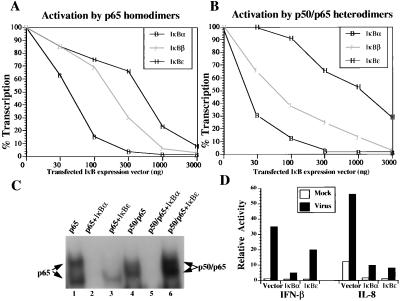
To investigate whether the interaction between IκBɛ and Rel proteins leads to a decrease in NF-κB transcriptional activity, we performed cotransfection experiments in mouse P19 cells that are devoid of endogenous NF-κB (18) by using the PRDII-CAT reporter that was activated by transfection of a small constant amount of the NF-κB p65 subunit along with increasing amounts of transfected IκBα-, IκBβ-, or IκBɛ-expressing plasmids. As shown in Fig. 3A, transfection of increasing amounts of IκBα, IκBβ, and IκBɛ decreased promoter activity in a dose-dependent manner. Consistent with our previous results, IκBα was a stronger inhibitor of NF-κB than IκBβ, because IκBβ is a weaker inhibitor of NF-κB DNA binding (14). Surprisingly, IκBɛ inhibited p65-dependent transcription only weakly, despite the fact that it is a strong inhibitor of p65 DNA binding in vitro (Fig. 2B). Similar results were obtained by using the p65/c-Rel heterodimer as an activator of transcription (data not shown). Unexpectedly, gene transcription activated by the p50/p65 heterodimer was only marginally inhibited by IκBɛ and only at very high concentrations of transfected IκBɛ-expressing plasmid, even though IκBɛ interacted with p50/p65 and efficiently decreased p50/p65 DNA binding in vitro (Fig. 2B). Similarly we showed that IκBɛ only weakly inhibited transcription activated by the p50/c-Rel heterodimer (data not shown).
Figure 3.
IκBɛ preferentially inhibits the p65 homodimeric form of NF-κB in vivo. (A) Mouse P19 cells were cotransfected with the PRDII-CAT reporter plasmid (200 ng) and a constant amount of p65 expression vector (100 ng) along with the indicated amounts of expression plasmids for IκBα, IκBβ, and IκBɛ. 100% transcription corresponds to the level of activation (67-fold) obtained in the absence of IκB plasmids. Shown is one of three independent experiments. The variability from experiment to experiment was less than 15%. (B) Same as in A, but the activator was an equimolar mixture of p50 and p65 expression vectors (100 ng). 100% corresponds to a 47-fold induction of transcription in the absence of IκB expression vectors. (C) Shown is an EMSA experiment using the PRDII oligonucleotide as a probe along with extracts prepared from COS cells transfected with the expression vectors indicated on the top of the gel. The identity of the complexes was verified by antibody supershift experiments (not shown). Transfection of p65 results in the formation of two complexes because of proteolytic cleavage during extract preparation. (D) Mouse 3T3 cells were cotransfected with the −110IFN-β-CAT or IL-8 luciferase (4 μg) along with IκBα or IκBɛ expression vectors (16 μg). Thirty-six hours after transfection the cells were infected with Sendai virus for 8 h, and the CAT and luciferase activities were determined.
To investigate in more detail this unusual property of IκBɛ we performed EMSA experiments with whole cell extracts prepared from COS cells cotransfected with p65 or p50/p65 expression plasmids along with IκBα or IκBɛ. As shown in Fig. 3C, coexpression of IκBα inhibited binding of both p65 heterodimers (compare lanes 1 and 2) and the p50/p65 heterodimer (compare lanes 4 and 5), a result which is in agreement with the experiments using recombinant proteins. In contrast, IκBɛ efficiently inhibited the p65 homodimer (lane 3) but not the p50/p65 heterodimer DNA binding in this assay (lane 6). Thus, in vivo, IκBɛ functions as an inhibitor specific to the p65 homodimers of Rel proteins (see also Discussion).
To address the biological significance of this observation we compared the ability of ectopically expressed IκBɛ to inhibit transcription activated by endogenous, induced NF-κB proteins from two different promoters known to be activated in vivo by distinct combinations of Rel proteins. We have previously shown that the PRDII binding site of the IFN-β gene promoter is optimally bound and activated by the p50/p65 heterodimer either in vivo or in vitro (18). On the other hand, the NF-κB site taken from the IL-8 promoter is optimally bound and activated by the p65 homodimers and not by the p50/p65 heterodimer (24). Mouse 3T3 cells were cotransfected with the −110IFN-β-CAT or the IL-8-luciferase reporters along with IκBα- or IκBɛ-expressing plasmids. The cells were either mock or virus infected for 8 h, and the CAT and luciferase activities were determined. Fig. 3D shows that IκBα is a strong inhibitor of both basal level and virus-induced transcription from both reporters, because IκBα inhibits in vivo both p65 homodimers and the p50/p65 heterodimer. In sharp contrast, IκBɛ was a strong inhibitor of the virus-induced transcription from the IL-8 promoter and not from the IFN-β promoter. This experiment rules out the possibility that IκBɛ is not efficiently expressed after transfection, because depending on the reporter used it can either inhibit or have no significant effect on NF-κB-dependent transcription. We conclude that IκBɛ affects only a subset of NF-κB-regulated genes in vivo such as those preferentially regulated by p65 homodimers (see Discussion).
IκBɛ Is a Weak Inhibitor of Nuclear NF-κB DNA Binding in Vivo.
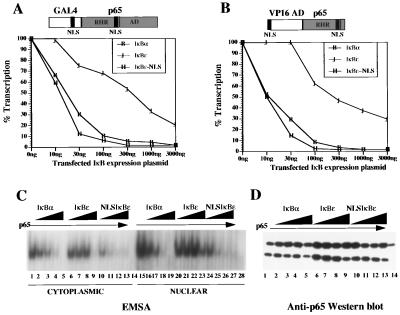
On the surface, the transfection experiments presented in Fig. 3A are inconsistent with the in vitro DNA binding data shown in Fig. 2B because IκBɛ is a strong inhibitor of the p65 homodimer DNA binding activity in vitro. To determine whether this apparent discrepancy is a consequence of the inability of IκBɛ to inhibit the nuclear NF-κB DNA binding activity in vivo, we carried out cotransfection experiments by using p65 derivatives containing, in addition to the RHR NLS, a second NLS derived from the SV40 T antigen or from the yeast activator GAL4. We have previously shown that either of these NLSs endows p65 with the ability to enter the nucleus even when it is complexed with the IκB proteins (14). Thus, in this assay any inhibition observed by IκBs on NF-κB proteins is not because of their cytoplasmic sequestration but rather because of an inhibition of their nuclear DNA binding (14). P19 cells were cotransfected with either the GAL4-p65 FL effector, which encodes the full-length p65 protein fused to the NLS and DNA-binding domain of GAL4, or with an effector that encodes a hybrid protein that bears the RHR and the NLS of p65 fused to the VP16 activation domain and the NLS from the SV40 T antigen, along with increasing amounts of IκBα- and IκBɛ-expressing plasmids. As shown in Fig. 4 A and B, coexpression of IκBα led to a significant decrease in transcription activated by either activator. In sharp contrast, IκBɛ only inhibited transcription weakly. This experiment suggested that the reason IκBɛ cannot efficiently inhibit the nuclear activity of NF-κB is because it cannot enter into the nucleus. To examine this possibility we fused the NLS from the SV40 T antigen to IκBɛ and determined the ability of this chimeric protein to inhibit nuclear NF-κB function. Fulfilling our prediction, coexpression of NLS-IκBɛ with either of the p65 chimeric activators resulted in a strong inhibition of p65-dependent transcriptional activation to levels comparable with IκBα (Fig. 4 A and B). Taken together, our experiments strongly suggest that in contrast to IκBα and IκBβ, IκBɛ cannot function in the nucleus to inhibit NF-κB DNA binding because it does not translocate into the nucleus efficiently.
Figure 4.
IκBɛ is a weak inhibitor of nuclear NF-κB DNA binding in vivo. (A) Mouse P19 cells were cotransfected with the PRDII-CAT reporter (200 ng) and the GAL4-p65FL activator (1 μg) (a diagrammatic structure is indicated on the top of the graph) along with increasing amounts of the indicated IκB expression vectors. 100% transcription corresponds to a 45-fold activation in the absence of IκB expression plasmids. Shown is one of three independent experiments; the variability from experiment to experiment was less that 20%. (B) Same as in A except the activator (100 ng) contained the RHR of p65 fused to the SV40 NLS and the VP16 activation domain, as shown on the top of the graph. 100% transcription corresponds to a 74-fold activation of transcription in the absence of IκBs. (C) Shown is an EMSA experiment performed with cytoplasmic (lanes 1–14) or nuclear extracts (lanes 15–28) derived from COS cells either untransfected (lanes 14 and 28, respectively) or cotransfected with p65 alone (lanes 1 and 15) or along with increasing amounts (1, 2, 4, and 8 μg) of IκBα (lanes 2–5 and 16–19), IκBɛ (lanes 6–9 and 20–23), and NLS-IκBɛ (lanes 10–13 and 24–28). (D) The same extracts shown in C were immunoblotted with a p65-specific antibody.
To provide additional evidence in support of this model we carried out mobility shift assays by using cytoplasmic and nuclear extracts prepared from COS cells transfected with p65 along with increasing amounts of IκBα, IκBɛ, and NLS-IκBɛ. As shown in Fig. 4C, untransfected COS cells do not have NF-κB DNA binding activity in either the cytoplasm or in the nucleus (lanes 14 and 28, respectively). However, transfection of the p65 expression plasmid resulted in the accumulation of DNA binding activity in both the cytoplasm and the nucleus (lanes 1 and 15, respectively). Cotransfection of increasing amounts of the IκBα expression plasmid inhibited p65 DNA binding derived from either the cytoplasm or the nucleus in a dose-dependent manner (lanes 2–5 and 16–19). Interestingly, coexpression of IκBɛ resulted in inhibition of the cytoplasmic NF-κB DNA binding activity (lanes 6–9) with no effect on nuclear NF-κB DNA binding (lanes 20–24). In addition, IκBɛ is a 2- to 4-fold weaker inhibitor of cytoplasmic NF-κB DNA binding when compared with IκBα (compare lanes 2–5 with 6–9), as we have observed with recombinant proteins (Fig. 2). Furthermore, this observation rules out the possibility that after transfection IκBα and IκBɛ are not expressed at comparable levels. Finally, overexpression of the NLS-IκBɛ protein efficiently inhibited both the nuclear and the cytoplasmic NF-κB DNA binding activity (lanes 24–27 and 10–13, respectively). To exclude the possibility that expression of the IκBs decreased the expression of p65 we performed the Western blot shown in Fig. 4D by using the same extracts immunoblotted with a p65-specific antibody. As shown in the figure, p65 expression was not affected by any of the IκBs (neither in the cytoplasm nor in the nucleus). In addition, the small inhibitory effect of IκBɛ on nuclear p65 DNA binding observed in Fig. 4C, lane 23, is most likely because of the lower levels of nuclear p65, as shown in the Western blot (Fig. 4D, compare lanes 8 and 9).
DISCUSSION
The activity of the transcription factor NF-κB is mainly regulated by the IκB proteins (reviewed in refs. 1–7). IκBs directly interact with NF-κB resulting in its cytoplasmic sequestration as well as in inhibition of NF-κB DNA binding. An extraordinarily large number of extracellular signals induces proteolytic degradation of IκBs and concomitant nuclear translocation of NF-κB. As there are multiple forms of NF-κB and IκB proteins complexed in the cytoplasm, we have been confronted with the question of whether these complexes are functionally redundant. However, recent studies have revealed that different combinations of Rel proteins display distinct transcriptional activities in different genes (18, 24, 25). In addition, the kinetics by which different Rel cytoplasmic complexes appear in the nucleus varies and depends on the cell type (26, 27). Finally, the inactivation by targeted gene disruption of individual Rel proteins in mice further demonstrated that there are distinct functions for different members of the Rel family (28–31). Similarly to the Rel proteins, the two different IκB proteins IκBα and IκBβ also have distinct functions in vivo (14, 15, 32). In this paper we describe the cloning and functional characterization of mouse IκBɛ, which shares several similar structural and functional properties with the other two IκBs (IκBα and IκBβ), but it appears to function primarily in the cytoplasm by inhibiting the nuclear translocation of p65 homodimers. In contrast, IκBα and IκBβ can also function in the nucleus to inhibit DNA binding of any Rel protein homo- or heterodimer containing p65 or c-Rel.
IκBɛ contains 6 ankyrin repeats that are highly homologous to the ankyrin repeats found in all other IκB proteins. However, there are two differences in the organization of the IκBɛ structural motifs. First, the region preceding the ankyrin repeats (amino terminus) is unusually long (120 aa), whereas the corresponding regions in IκBα and IκBβ are much shorter. In that respect, IκBɛ resembles Cactus, the Drosophila homologue of mammalian IκBs (33). However, as is the case for IκBα and IκBβ, the amino terminus of IκBɛ contains a conserved pair of serine residues, the signal-responsive domain, that is required for its inducible degradation in response to extracellular signals (10). The second difference is the relatively short carboxyl terminus of IκBɛ compared with IκBα and IκBβ. In IκBα and IκBβ, this domain is acidic and contains a PEST region, which has been implicated in basal turnover and in inducible degradation (21–23). In contrast, the carboxyl terminus of IκBɛ does not contain a typical PEST region. However, the IκBɛ carboxyl terminus is acidic, and we showed that it is required for efficient inhibition of NF-κB DNA binding as is the case for IκBα and IκBβ (14). In addition, the carboxyl terminus of IκBɛ contains a consensus casein kinase II (CKII) site (multiple CKII sites have been mapped in IκBα and IκBβ), and we have shown that CKII phosphorylates IκBɛ in vitro at this site (data not shown).
Another common property shared by IκBɛ, IκBα, and IκBβ is their ability to inhibit NF-κB DNA binding. We showed that recombinant and unphosphorylated IκBɛ removes p65 homodimers or p50/p65 heterodimers from DNA as efficiently as IκBα does. In sharp contrast, recombinant IκBβ is a poor inhibitor of NF-κB DNA binding, but IκBβ phosphorylated by CKII becomes a strong inhibitor of NF-κB DNA binding (14). The biological significance of this property of IκBα and IκBβ is that both proteins can enter the nucleus to terminate NF-κB-dependent transcriptional activation (14). Given the strong inhibitory effect of IκBɛ on NF-κB DNA binding, we were surprised to find that in transfection experiments IκBɛ was a weaker inhibitor of NF-κB than IκBα or even IκBβ. We showed that IκBɛ is a 10- and 3-fold weaker inhibitor than IκBα and IκBβ, respectively, of p65 homodimer-dependent transcriptional activation. This difference is not because of lower levels of IκBɛ expression but rather because of the inability of IκBɛ to enter the nucleus and inhibit p65 DNA binding. However, when we fused the SV40 NLS to IκBɛ we observed a strong inhibition of the nuclear p65 transcriptional activity. These experiments functionally distinguish IκBɛ from IκBα and IκBβ. This is because we have previously shown that both IκBα and IκBβ inhibit NF-κB by a combination of cytoplasmic sequestration and by their ability to function as postinduction repressors of NF-κB by entering the nucleus and removing NF-κB from the promoters (14). This conclusion is also supported by the fact that in cells lacking IκBα, the NF-κB DNA binding activity persists for several hours following induction (34), despite the fact that the IκBɛ protein levels are up-regulated in these cells (10). We believe that the elevated levels of IκBɛ protein in the cytoplasm compensate for the lack of IκBα, so there is no constitutive NF-κB in the nucleus, but IκBɛ cannot enter the nucleus to inhibit NF-κB DNA binding and shut off transcription. At present, we do not understand why IκBɛ does not translocate into the nucleus efficiently. In the IκBα case, nuclear translocation is due perhaps to a simple diffusion into the nucleus, as IκBα is of low molecular mass (37 kDa) and is synthesized at high amounts after its degradation (13). On the other hand, the nuclear translocation of IκBβ (45 kDa) depends on its phosphorylation status, presumably by CKII (14, 15, 32). In this case, newly synthesized and unphosphorylated IκBβ enters the nucleus whereas the phosphorylated form remains in the cytoplasm. Because IκBɛ has a similar size to IκBβ and is phosphorylated in vivo, it is conceivable to assume that IκBɛ may also be regulated in a similar manner where constitutive phosphorylation by CKII or other kinases restricts its nuclear translocation. In summary, our experiments strongly suggest that the main inhibitory activity of IκBɛ on NF-κB is because of cytoplasmic sequestration and not because of inhibition of NF-κB DNA binding in the nucleus.
The most unusual property of IκBɛ is its inability to inhibit transcription from the p50/p65 heterodimer, which is the most abundant form of inducible NF-κB. It should be emphasized here that when purified p50/p65 and IκBɛ were used in EMSA experiments, IκBɛ was a strong inhibitor of DNA binding. However, IκBɛ did not inhibit p50/p65 DNA binding when all these components were transfected in COS cells. This result is in agreement with the observation that in vivo IκBɛ is predominantly found associated with p65 homodimers and to a lesser extent with p65/c-Rel heterodimers (10). On the other hand, the majority of IκBα and IκBβ is associated with the p50/p65 heterodimer (7, 10). At present, we do not understand the reason for this high level of specificity, but clearly it is not because of the inability of IκBɛ to interact with the p50/p65 heterodimer. However, we can imagine that this interaction is of low affinity and below the threshold needed when other proteins are present or that posttranslational modifications in either p50/p65 or IκBɛ affect this interaction or that other proteins inhibit this interaction in vivo. The most significant implication from this observation is that IκBɛ would function as a highly specific inhibitor of genes regulated preferentially by p65 homodimers. A dramatic illustration of this idea was demonstrated when we compared the effects of IκBα and IκBɛ on the expression of two different genes previously known to be optimally activated by the p50/p65 heterodimer and p65 homodimers, respectively. The PRDII site from the IFN-β gene is bound by both the p50/p65 heterodimer and p65 homodimers, but the former is the optimal activator following virus induction (18). On the other hand, the NF-κB site from the IL-8 gene is bound and activated by p65 homodimer and not by the p50/p65 heterodimer (24). We showed that IκBα inhibits virus induction very efficiently from both reporters, because it can inhibit both p50/p65 heterodimers and the p65 homodimers. In contrast, IκBɛ only inhibited transcription efficiently from the IL-8 promoter and had a small effect on the expression of the IFN-β gene. Thus, IκBɛ functions in vivo as a gene-specific inhibitor of NF-κB proteins.
The results presented in this paper taken together with previous studies on IκB proteins indicate that there is a clear division of labor among IκBα, IκBβ, and IκBɛ. Depending on the cell type, the majority of cytoplasmic bona fide NF-κB (p50/p65 heterodimer) is associated with IκBα and IκBβ, whereas IκBɛ is responsible for restricting the p65 homodimers in the cytoplasm. Following cell stimulation and depending on the cell type and the inducer, some or all of the IκBs are degraded with different kinetics, resulting in the nuclear accumulation of different homo- and heterodimeric forms of Rel proteins to activate transcription. Among the targets induced by NF-κB are the genes encoding IκBα and IκBɛ. The newly synthesized IκB proteins have different functions. IκBα reassociates in the cytoplasm with the p50/p65 heterodimer and also enters the nucleus to remove NF-κB from actively transcribed genes terminating the response (14). IκBɛ cannot enter the nucleus and is associated in the cytoplasm with p65 homodimers or p65/c-Rel heterodimers. If the inducer caused the degradation of IκBβ, then the newly synthesized and unphosphorylated IκBβ enters the nucleus and forms a ternary complex with NF-κB on certain NF-κB sites [those that do not bind HMG I(Y)], rendering these NF-κB molecules refractory to inhibition by nuclear IκBα (14, 15). Thus, the existence of different members of Rel and IκB proteins in conjunction with their differential response to extracellular signals and their differential ability to influence the function of each other provide part of the specificity required for the development and differentiation of cells in response to extracellular signals.
Acknowledgments
We thank S. Goff (Columbia University) for the yeast expression library, K. Leclair (Harvard University) for the IL-8 luciferase plasmid, I. Sadowski (University of British Columbia) for the PMVN vectors, T. C. Gilliam (Columbia University) for a mouse ZAP II mouse cDNA library, and members of the laboratory for useful comments during the course of the experiments. This work was supported by an American Heart Association grant-in-aid, the Pew Scholars Program in Biomedical Sciences, the Irma T. Hirschl Foundation, the Council for Tobacco Research-USA Inc., and in part by National Institutes of Health Grant 1 RO1GM54605-01). S.L. was supported by the National Institutes of Health Training Grant 2T32DK07328.
ABBREVIATIONS
- RHR
Rel homology region
- NLS
nuclear localization signal
- TNF-α
tumor necrosis factor-α
- IL-1
interleukin 1
- PMA
phorbol 12-myristate 13-acetate
- EMSA
electrophoretic mobility shift assays
- GST
glutathione S-transferase
- CAT
chloramphenicol acetyltransferase
- IFN-β
interferon-β
- CKII
casein kinase II
Footnotes
The sequence reported in this paper has been deposited in the GenBank database (accession no. AF030896).
References
- 1.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S., Jr Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Siebenlist U, Franzoso G, Brown K. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 4.Thanos D, Maniatis T. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle P A, Baltimore D. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Baldwin A S., Jr Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 7.Verma I, Stevenson J K, Schwarz E M, Antwerp D V, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 8.Haskill S, Beg A A, Tompkins S M, Morris J S, Yurochko A D, Sampson-Johanne A, Mondal K, Ralph P, Baldwin A S. Cell. 1991;65:1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- 9.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside S T, Epinat J-C, Rice N R, Israel A. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. Cell. 1993;72:729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Nolan G P, Liu H C, Scott M L, Baltimore D. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 13.Sun S C, Ganchi P A, Ballard D W, Greene W C. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 14.Tran K, Merika M, Thanos D. Mol Cell Biol. 1997;17:5386–5399. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suyang H, Phillips R, Douglas I, Ghosh S. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadowski I, Bell B, Broad P, Hollis M. Gene. 1992;18:137–141. doi: 10.1016/0378-1119(92)90261-m. [DOI] [PubMed] [Google Scholar]
- 17.Liang S D, Marmonstein R, Harrison S C, Ptashne M. Mol Cell Biol. 1996;16:3773–3781. doi: 10.1128/mcb.16.7.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanos D, Maniatis T. Mol Cell Biol. 1995;15:152–164. doi: 10.1128/mcb.15.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning, A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 7.39–7.52. [Google Scholar]
- 20.Andrews N C, Faller D V. Nucleic Acids Res. 1991;19:2449. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beauparlant P, Lin R, Hiscott J. J Biol Chem. 1996;271:10690–10696. doi: 10.1074/jbc.271.18.10690. [DOI] [PubMed] [Google Scholar]
- 22.Ernst M K, Dunn L L, Rice N. Mol Cell Biol. 1995;15:872–882. doi: 10.1128/mcb.15.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Antwerp D J, Verma I M. Mol Cell Biol. 1996;16:6037–6045. doi: 10.1128/mcb.16.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunsch C, Rosen C A. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins N D, Schmid R M, Duckett C S, Leung K, Rice N R, Nabel G J. Proc Natl Acad Sci USA. 1992;89:1529–1533. doi: 10.1073/pnas.89.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou H C, Sha W, Scott M, Baltimore D. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard D W, Walker W H, Doerre S, Sista P, Molitor J A, Dixon E P, Peffer N J, Hennick M, Greene W C. Cell. 1990;63:803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- 28.Sha W, Liou H C, Tuomanen E, Baltimore D. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 29.Weih F, Carrasco D, Durham S, Barton D, Rizzo C, Ryseck R P, Lira S, Bravo R. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 30.Burkly L, Hession C, Ogata L, Reilly C, Marconi L, Olson D, Tizard R, Cate R, Lo D. Nature (London) 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 31.Beg A, Sha W, Bronson R, Ghosh S, Baltimore D. Nature (London) 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 32.Chu Z-L, McKinsey T A, Liu L, Qi X, Ballard D W. Mol Cell Biol. 1996;16:5974–5984. doi: 10.1128/mcb.16.11.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steward R. Science. 1987;238:692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- 34.Beg A A, Sha W C, Bronson R T, Baltimore D. Genes Dev. 1996;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]