Abstract
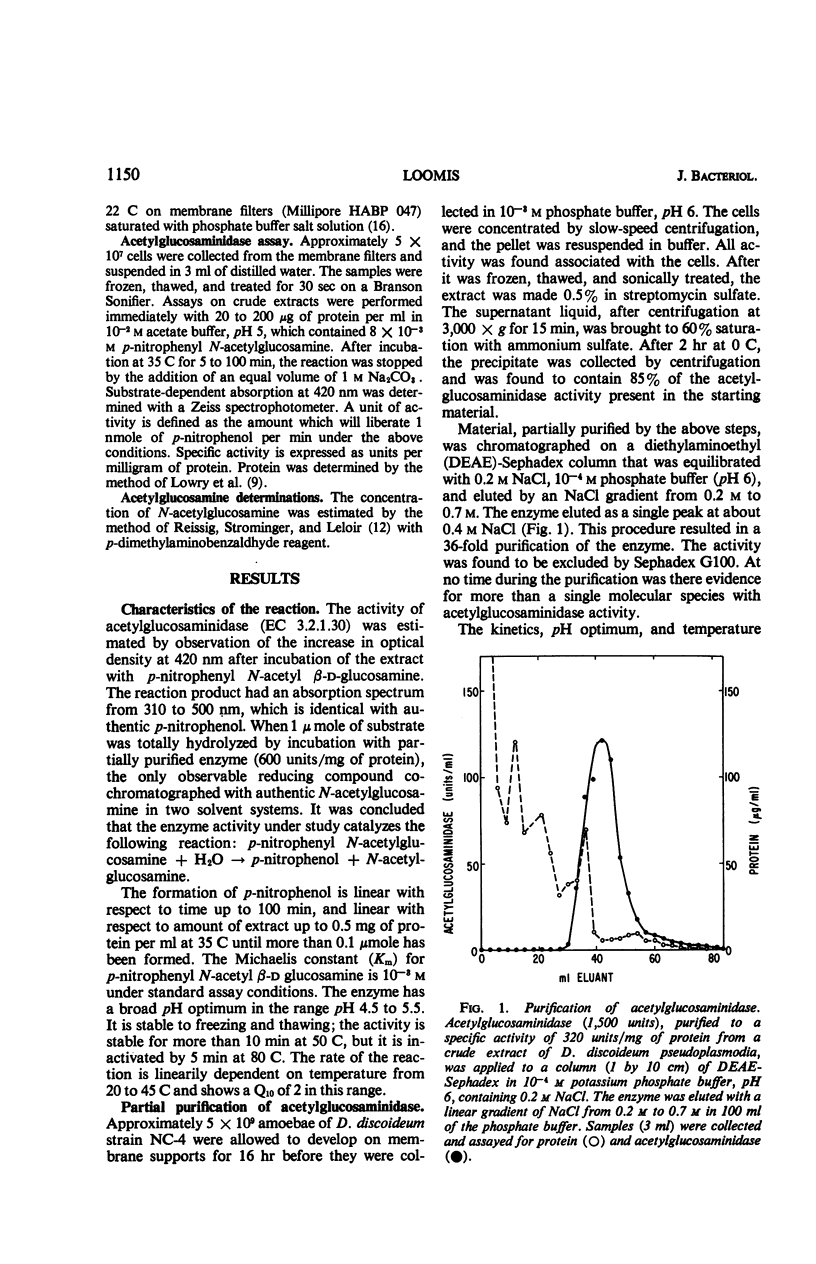
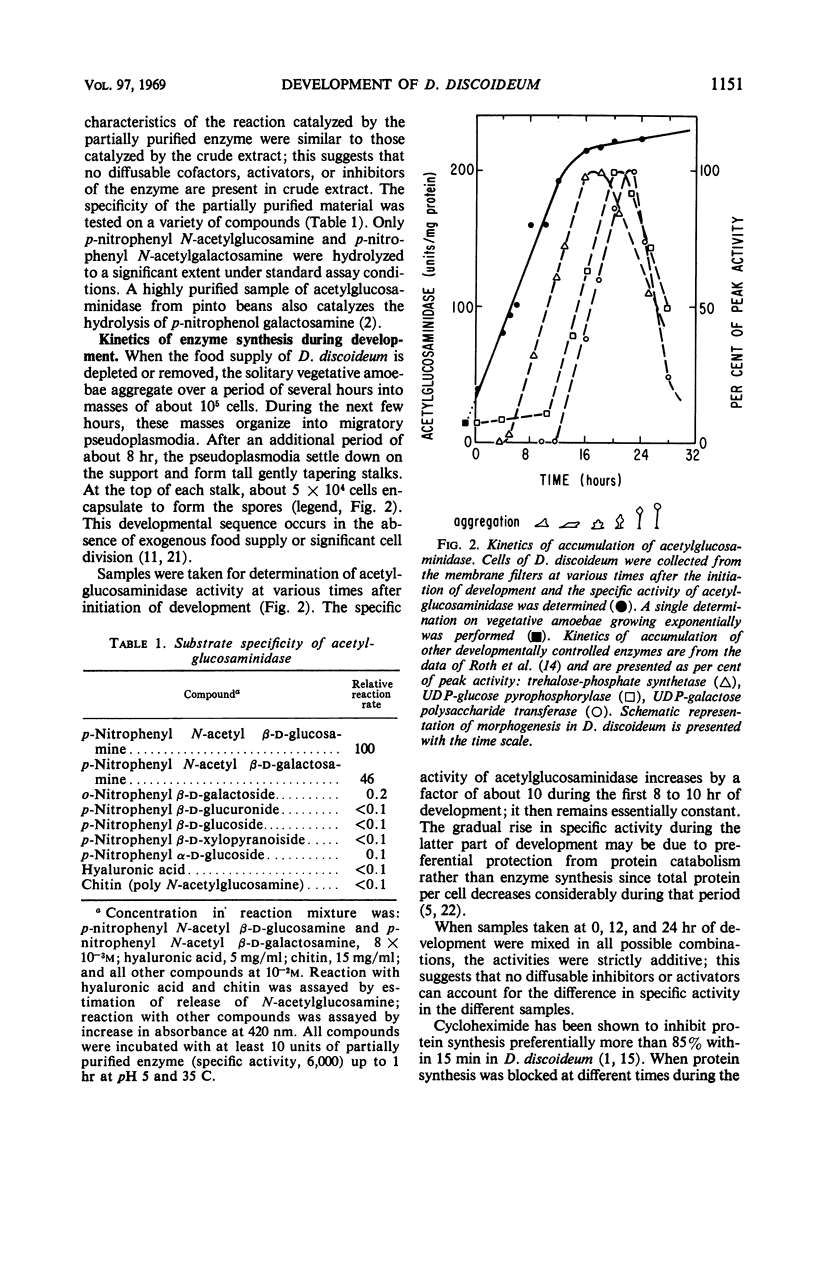
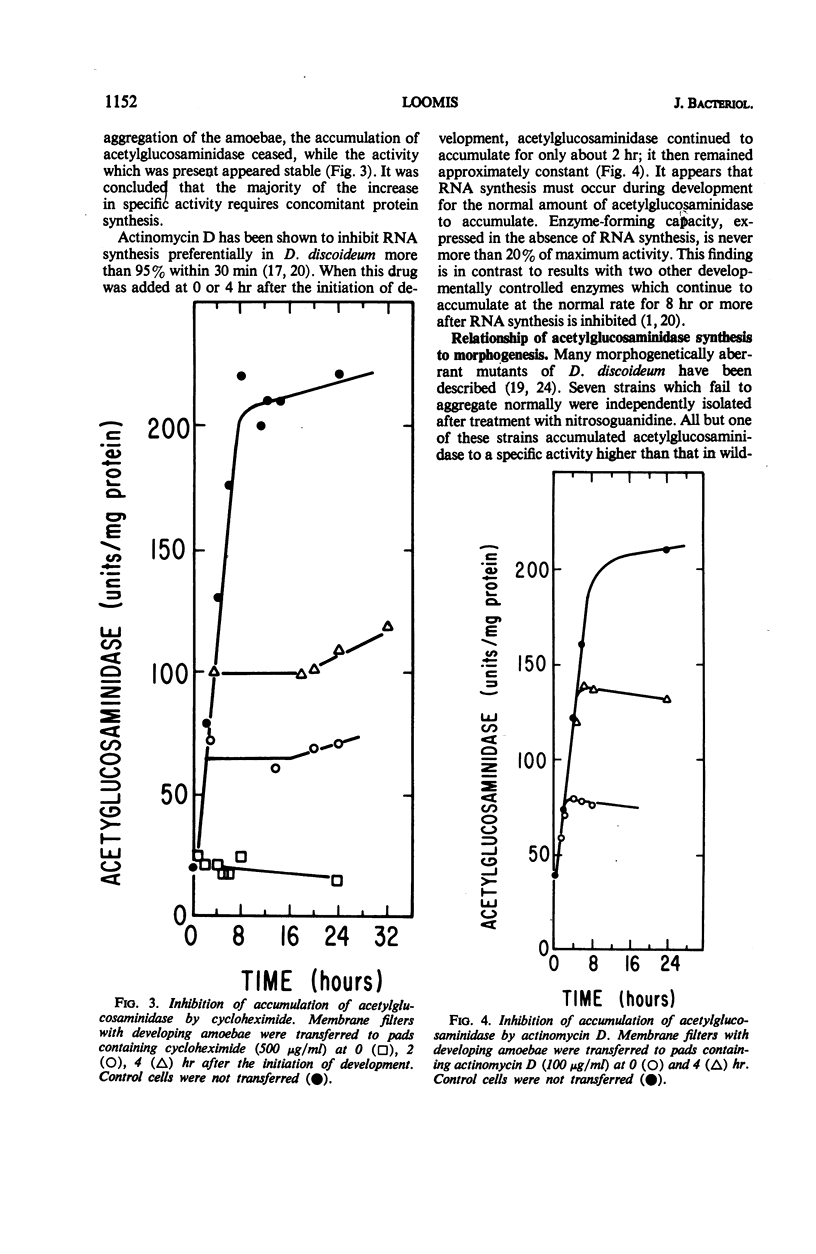
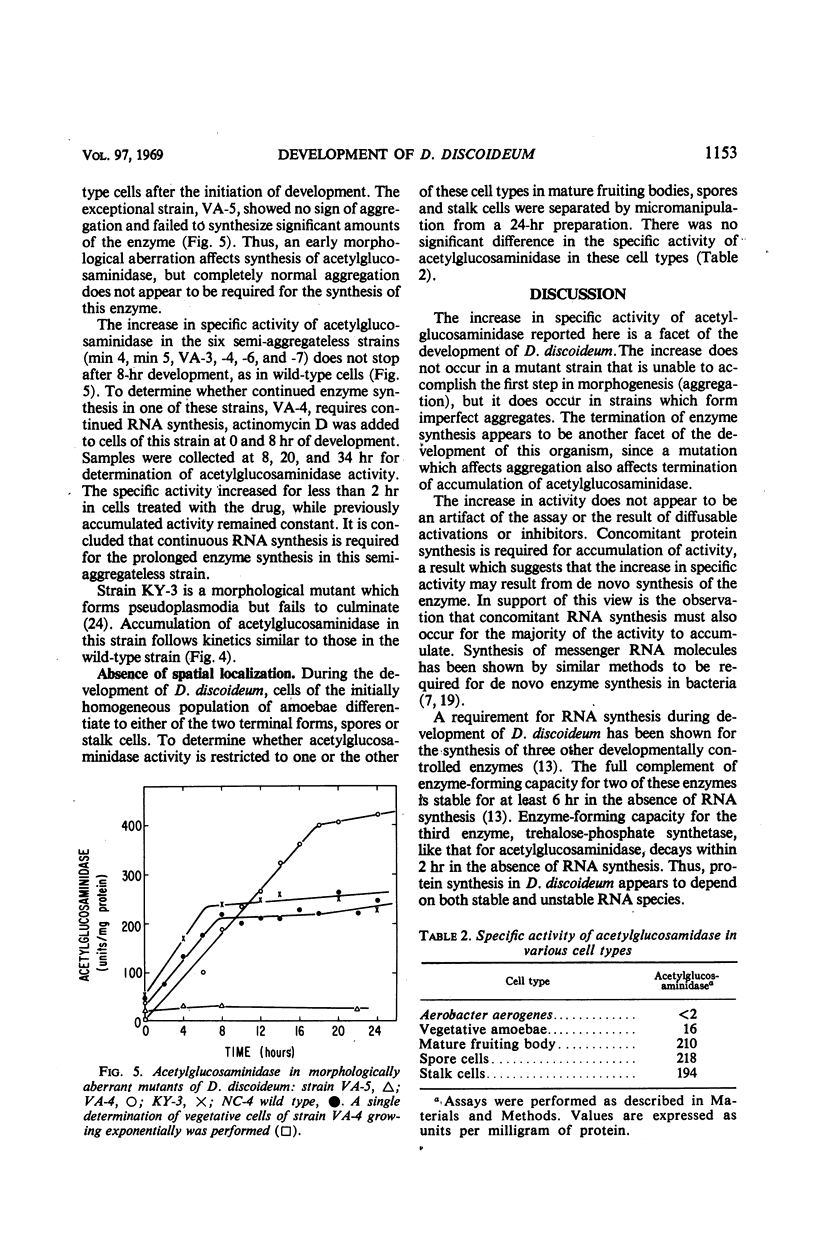
The specific activity of acetylglucosaminidase has been found to increase more than 10-fold during the first 10 hr of development in the cellular slime mold Dictyostelium discoideum. The specific activity then remained essentially constant until after germination. The activity was purified 36-fold and found to behave as a single protein species. The increase in specific activity required concomitant protein synthesis. If ribonucleic acid synthesis was preferentially inhibited during the period of synthesis of acetylglucosaminidase, further increase in enzymatic activity stopped after 2 hr. The increase in activity did not occur in a mutant strain which did not undergo the first step in morphogenesis. Mutant strains, blocked slightly later in morphogenesis, synthesized the enzyme at the normal rate but for an extended period. It was concluded that the initiation and termination of synthesis of acetylglucosaminidase are controlled by the developmental program.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Sussman M. The appearance and disappearance of uridine diphosphate glucose pyrophosphorylase activity during differntiation of the cellular slime mold Dictyostelium discoideum. J Biol Chem. 1967 Apr 25;242(8):1696–1700. [PubMed] [Google Scholar]
- Bahl O. P., Agrawal K. M. Glycosidases of Phaseolus vulgaris. I. Isolation and characterization of beta-N-acetylglucosaminidase. J Biol Chem. 1968 Jan 10;243(1):98–102. [PubMed] [Google Scholar]
- Cleland S. V., Coe E. L. Activities of glycolytic enzymes during the early stages of differentiation in the cellular slime mold Dictyostelium discoideum. Biochim Biophys Acta. 1968 Feb 1;156(1):44–50. doi: 10.1016/0304-4165(68)90102-5. [DOI] [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MECHANISM OF HISTIDASE INDUCTION AND FORMATION IN BACILLUS SUBTILIS. J Mol Biol. 1964 Oct;10:105–119. doi: 10.1016/s0022-2836(64)80031-0. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Ashworth J. M. Plaque-size mutants of the cellular slime mould Dictyostelium discoideum. J Gen Microbiol. 1968 Sep;53(2):181–186. doi: 10.1099/00221287-53-2-181. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Roth R., Ashworth J. M., Sussman M. Periods of genetic transcription required for the synthesis of three enzymes during cellular slime mold development. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1235–1242. doi: 10.1073/pnas.59.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Sussman M. Trehalose 6-phosphate synthetase (uridine diphosphate glucose: d-glucose 6-phosphate 1-glucosyltransferase) and its regulation during slime mold development. J Biol Chem. 1968 Oct 10;243(19):5081–5087. [PubMed] [Google Scholar]
- SUSSMAN M., OSBORN M. J. UDP-GALACTOSE POLYSACCHARIDE TRANSFERASE IN THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM: APPEARANCE AND DISAPPEARANCE OF ACTIVITY DURING CELL DIFFERENTIATION. Proc Natl Acad Sci U S A. 1964 Jul;52:81–87. doi: 10.1073/pnas.52.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSSMAN R. R., SUSSMAN M. Cellular differentiation in dictyosteliaceae; heritable modifications of the developmental pattern. Ann N Y Acad Sci. 1953 Oct 14;56(5):949–960. doi: 10.1111/j.1749-6632.1953.tb30274.x. [DOI] [PubMed] [Google Scholar]
- Sussman M., Loomis W. F., Jr, Ashworth J. M., Sussman R. R. The effect of actinomycin D on cellular slime mold morphogenesis. Biochem Biophys Res Commun. 1967 Feb 8;26(3):353–359. doi: 10.1016/0006-291x(67)90131-3. [DOI] [PubMed] [Google Scholar]
- Sussman M., Sussman R. R. The regulatory program for UDPgalactose polysaccharide transferase activity during slime mold cytodifferentiation: requirement for specific synthesis of ribonucleic acid. Biochim Biophys Acta. 1965 Nov 8;108(3):463–473. doi: 10.1016/0005-2787(65)90038-9. [DOI] [PubMed] [Google Scholar]
- WHITE G. J., SUSSMAN M. Metabolism of major cell components during slime mold morphogenesis. Biochim Biophys Acta. 1961 Oct 28;53:285–293. doi: 10.1016/0006-3002(61)90441-3. [DOI] [PubMed] [Google Scholar]
- Wright B. E. Multiple causes and controls in differentiation. Science. 1966 Aug 19;153(3738):830–837. doi: 10.1126/science.153.3738.830. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K., Loomis W. F., Jr, Sussman M. Developmental regulation of the enzyme UDP-galactose polysaccharide transferase. Exp Cell Res. 1967 May;46(2):328–334. doi: 10.1016/0014-4827(67)90070-5. [DOI] [PubMed] [Google Scholar]


