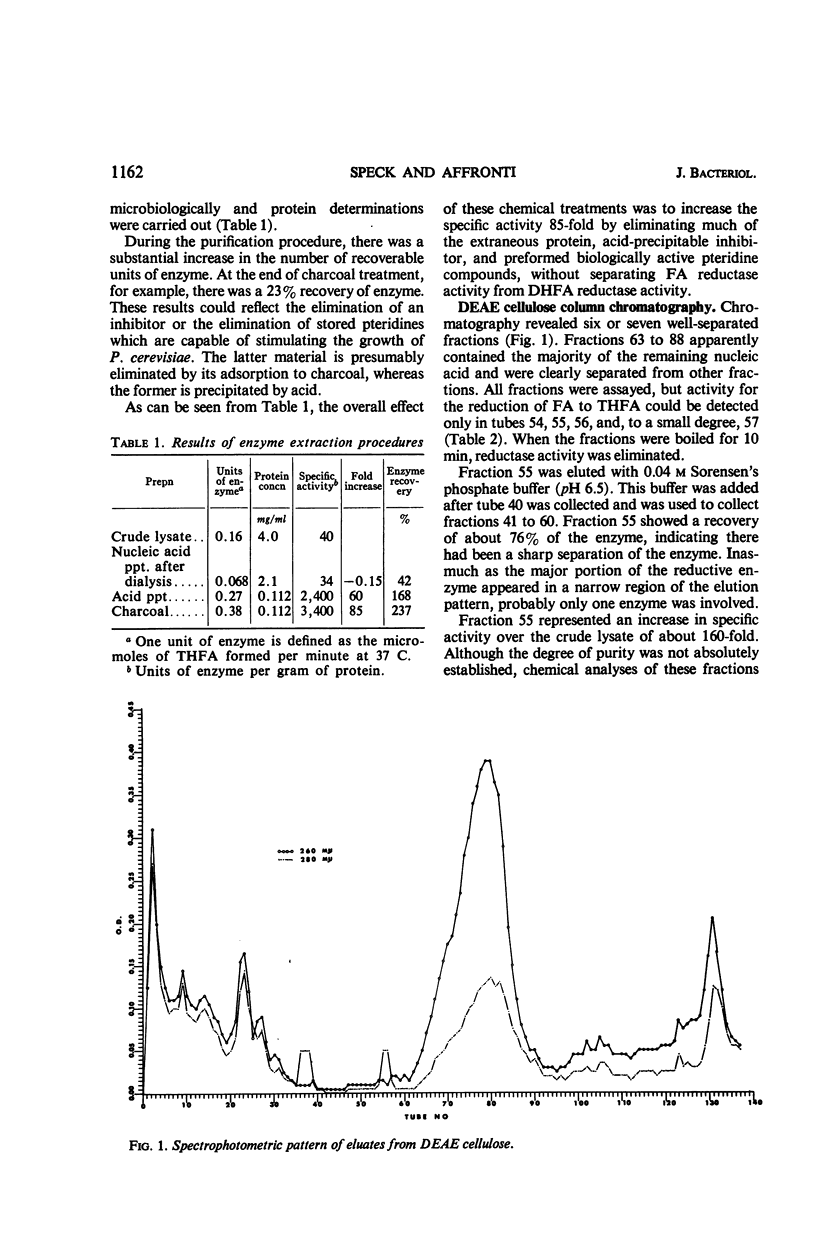
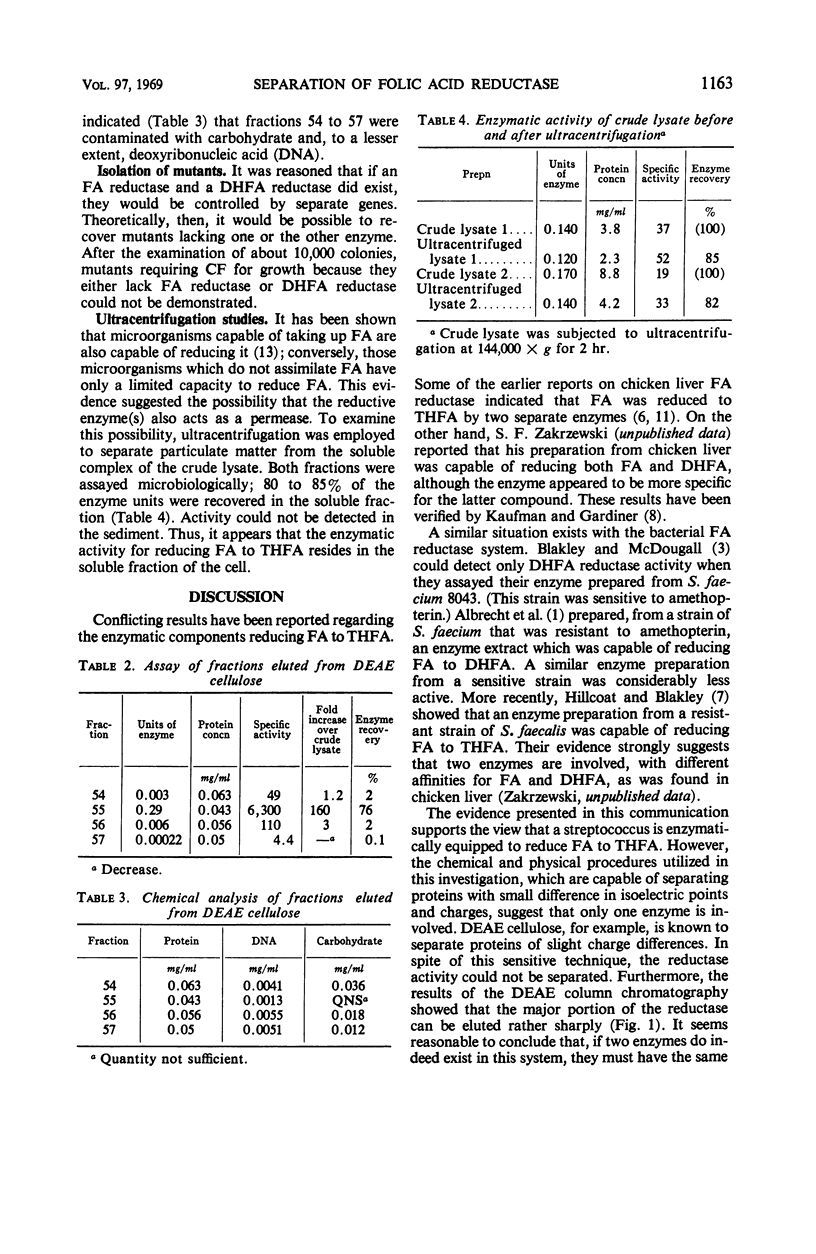
Abstract
A strain of Streptococcus faecium (ATCC 8043) which is highly resistant to the antifolic acid compound, amethopterin, was gently ruptured by exposing protoplasts of the organism to a hypotonic solution. The crude lysate resulting there-from was treated by various chemical and physical techniques designed to separate folic acid reductase from dihydrofolic acid reductase. In the process, the enzyme was purified approximately 160-fold; however, throughout the process, the enzyme preparation maintained the ability to reduce folic acid to tetrahydrofolic acid. Attempts to isolate mutants showing a deficiency in either folic acid reductase or dihydrofolic acid reductase were unsuccessful. Based on these results, it is concluded that folic acid is reduced to tetrahydrofolic acid by one enzyme in S. faecium (ATCC 8043). The crude lysate was also subjected to ultracentrifugation. An analysis of the supernatant fluid and the sediment indicated that the reductive activity is located in the soluble fraction of the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht A. M., Palmer J. L., Hutchison D. J. Differentiating properties of the dihydrofolate reductases of amethopterin-resistant Streptococcus faecalis/Ak and the sensitive parent strain. J Biol Chem. 1966 Mar 10;241(5):1043–1048. [PubMed] [Google Scholar]
- BAKERMAN H. A. A method for measuring the microbiological activity of tetrahydrofolic acid and other labile reduced folic acid derivatives. Anal Biochem. 1961 Dec;2:558–567. doi: 10.1016/0003-2697(61)90023-9. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., LAKE D. E., NIVEN C. F., Jr PHYSIOLOGY OF THE ENTEROCOCCI AS RELATED TO THEIR TAXONOMY. J Bacteriol. 1963 Dec;86:1275–1282. doi: 10.1128/jb.86.6.1275-1282.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- Hillcoat B. L., Blakley R. L. Dihydrofolate reductase of Streptococcus faecalis. I. Purification and some properties of reductase from the wild strain and from strain A. J Biol Chem. 1966 Jul 10;241(13):2995–3001. [PubMed] [Google Scholar]
- Kaufman B. T., Gardiner R. C. Studies on dihydrofolic reductase. I. Purification and properties of dihydrofolic reductase from chicken liver. J Biol Chem. 1966 Mar 25;241(6):1319–1328. [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., HUENNEKENS F. M. Enzymatic reduction of dihydrofolic acid. J Biol Chem. 1958 Oct;233(4):969–974. [PubMed] [Google Scholar]
- WOOD R. C., FERONE R., HITCHINGS G. H. The relationship of cellular permeability to the degree of inhibition by amethopterin and pyrimethamine in several species of bacteria. Biochem Pharmacol. 1961 May;6:113–124. doi: 10.1016/0006-2952(61)90155-1. [DOI] [PubMed] [Google Scholar]
- WOOD R. C., WISE M. F. CONVERSION OF FOLIC ACID DERIVATIVES TO THEIR CONJUGATED FORMS BY CELLS OF STREPTOCOCCUS FAECALIS. Tex Rep Biol Med. 1965;23:512–529. [PubMed] [Google Scholar]


