Abstract
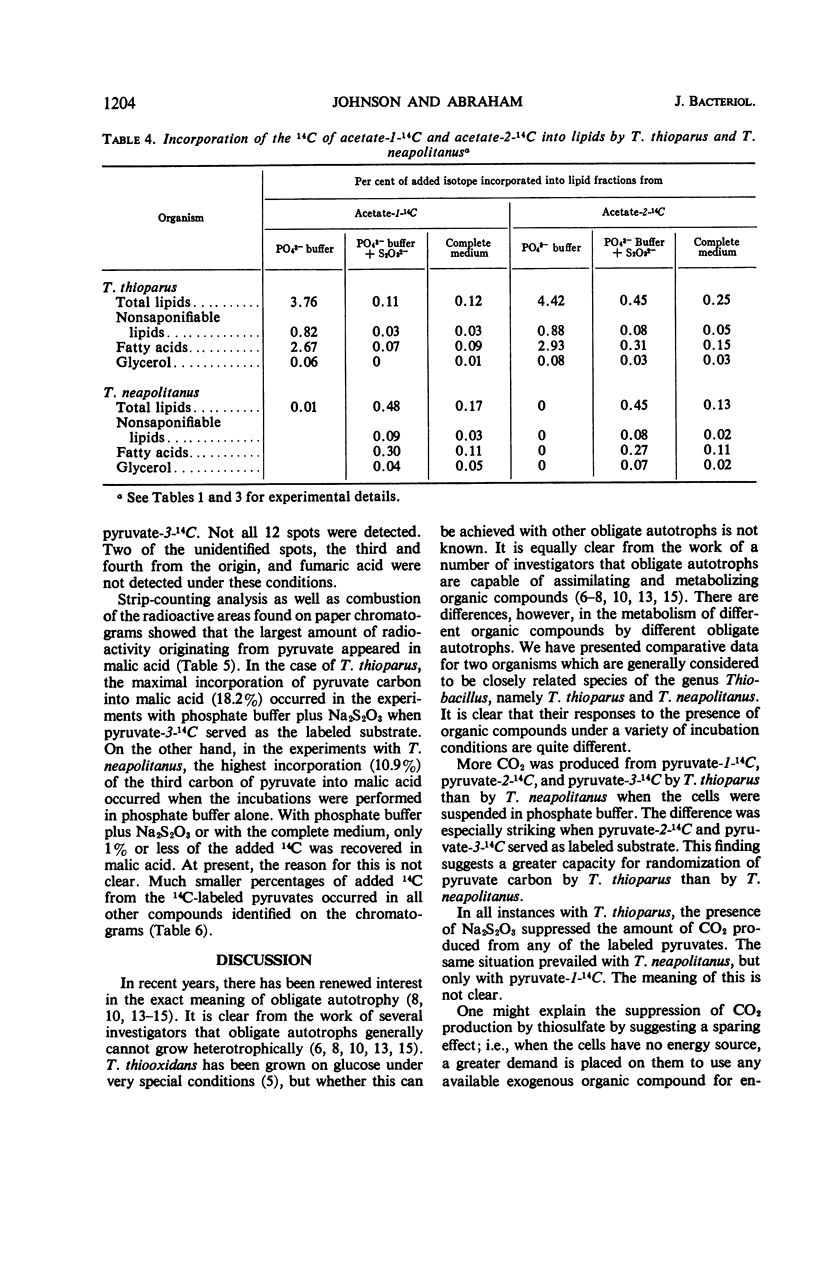
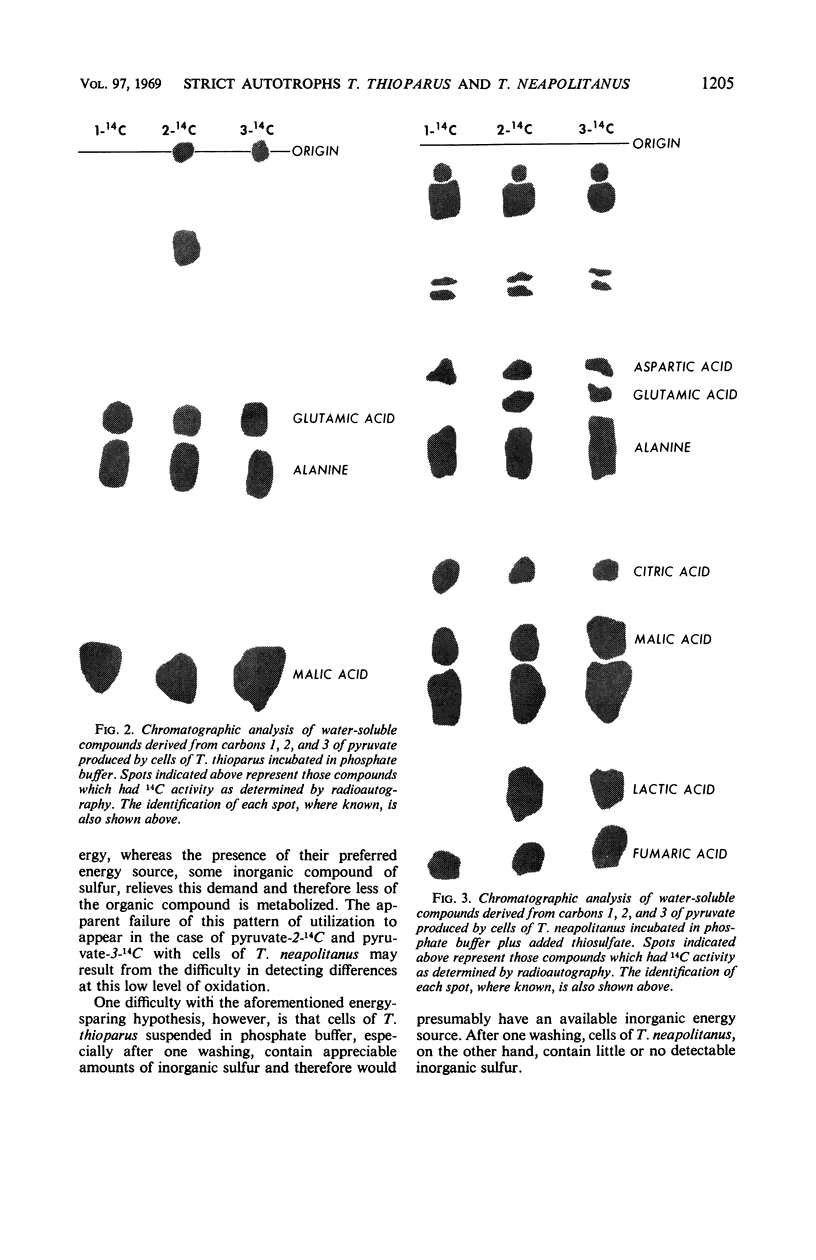
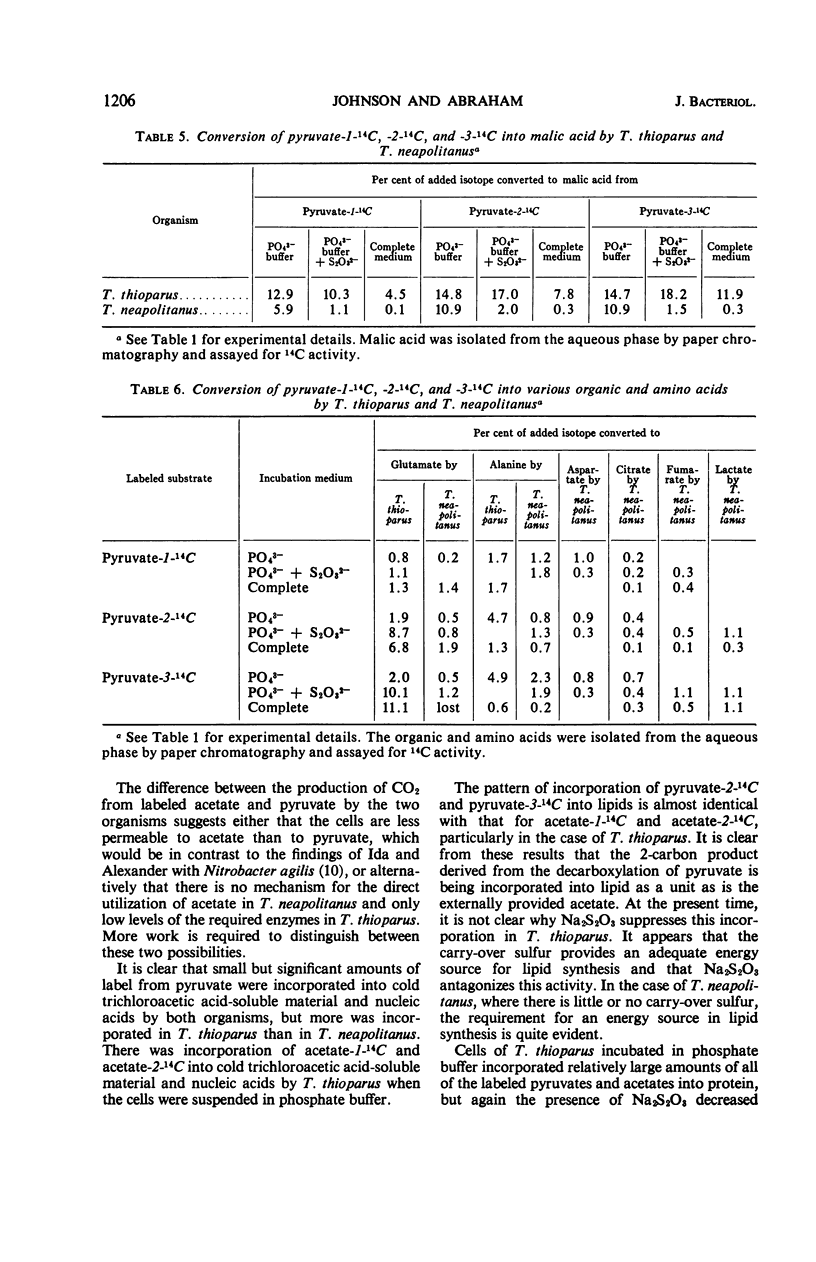
The assimilation and utilization of the individual carbon atoms of pyruvate and acetate by cells of Thiobacillus thioparus and T. neapolitanus, in the presence and absence of an energy source, were studied by use of radioactive substrates. Both organisms produced 14CO2 from 14C-labeled pyruvate, but more came from carbon 1 than from carbons 2 or 3. The conversion of the carbons of acetate to CO2 by both organisms was much less than that from any of the pyruvate carbons. When labeled pyruvate and acetate were incubated with these organisms, small amounts of radioactivity were found in the tricholoacetic acid-soluble material, nucleic acids, and lipids, and larger amounts were found in the protein fraction. The composition of the incubation medium affected the amount of utilization and incorporation of labeled substrates by both organisms. The presence of an exogenous energy source (Na2S2O3) suppressed incorporation of the labeled substrates into various cellular components by T. thioparus, but enhanced incorporation by T. neapolitanus. When 14C-pyruvate was used as a substrate, as many as 12 radioactive compounds were found in the water-soluble fraction in the experiments with T. neapolitanus, whereas no more than three radioactive compounds were detected in this fraction in the experiments with T. thioparus. Of the total 14C activity found in the water-soluble fractions, malic acid contained the highest percentage. These findings are discussed in light of the overall metabolism of these two sulfur-oxidizing obligate chemoautotrophs, as well as in relation to the biochemical basis of chemoautotrophy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartley J. C., Abraham S. Improved methods for liquid scintillation assay of (A) C14-compounds on paper chromatograms and (B) C14-protein. Adv Tracer Methodol. 1966;3:69–79. doi: 10.1007/978-1-4684-8625-4_9. [DOI] [PubMed] [Google Scholar]
- Borichewski R. M. Keto acids as growth-limiting factors in autotrophic growth of Thiobacillus thiooxidans. J Bacteriol. 1967 Feb;93(2):597–599. doi: 10.1128/jb.93.2.597-599.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borichewski R. M., Umbreit W. W. Growth of Thiobacillus thiooxidans on glucose. Arch Biochem Biophys. 1966 Sep 26;116(1):97–102. doi: 10.1016/0003-9861(66)90017-8. [DOI] [PubMed] [Google Scholar]
- Butler R. G., Umbreit W. W. Absorption and utilization of organic matter by the strict autotroph, Thiobacillus thiooxidans, with special reference to aspartic acid. J Bacteriol. 1966 Feb;91(2):661–666. doi: 10.1128/jb.91.2.661-666.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Effect of mixed culture on Nitrosomonas europaea simulated by uptake and utilization of pyruvate. J Bacteriol. 1966 Jan;91(1):367–373. doi: 10.1128/jb.91.1.367-373.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Finstein M. S. Carbon and Energy Sources for the Nitrifying Autotroph Nitrobacter. J Bacteriol. 1965 Jul;90(1):102–107. doi: 10.1128/jb.90.1.102-107.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. J., Jr, Morita Y., Mendelsohn L. Incorporation in vitro of precursor into protein and RNA of rat adrenal glands. Endocrinology. 1967 Mar;80(3):521–526. doi: 10.1210/endo-80-3-521. [DOI] [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Influence of amino acids and organic antimetabolites on growth and biosynthesis of the chemoautotroph Thiobacillus neapolitanus strain C. Arch Mikrobiol. 1967 Feb 20;56(2):91–105. doi: 10.1007/BF00408761. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Problems of the autotrophic micro-organisms. Sci Prog. 1967 Spring;55(217):35–51. [PubMed] [Google Scholar]
- London J., Rittenberg S. C. Effects of organic matter on the growth of Thiobacillus intermedius. J Bacteriol. 1966 Mar;91(3):1062–1069. doi: 10.1128/jb.91.3.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux J. V., Johnson E. J. Effect of adenosine monophosphate, adenosine diphosphate, and reduced nicotinamide adenine dinucleotide on adenosine triphosphate-dependent carbon dioxide fixation in the autotroph Thiobacillus neapolitanus. J Bacteriol. 1967 Aug;94(2):409–414. doi: 10.1128/jb.94.2.409-414.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VONKORFF R. W. PYRUVATE-C14, PURITY AND STABILITY. Anal Biochem. 1964 Jun;8:171–178. doi: 10.1016/0003-2697(64)90043-0. [DOI] [PubMed] [Google Scholar]



