Abstract
Acid phosphatase of Staphylococcus aureus PS55 was eluted from the surface of these cells with 1.0 m KCl at pH 8.5 by gentle agitation at 25 C and was purified 44-fold (51% recovery) by two cycles of dialysis and gel filtration. The eluted enzyme which had a 280/260 (nm) absorbancy ratio of 0.71 required at least 0.5 m salt solution for solubilization; however, most of the purified product which had a 280/260 (nm) absorbancy ratio of 1.72 was soluble in dilute buffer solution [0.01 m tris(hydroxymethyl)aminomethane chloride, pH 8.5]. Purified acid phosphatase appeared homogeneous according to the criteria of gel filtration, starch-block electrophoresis, and analytical ultracentrifugation. In a starch block, migration was toward the cathode at pH 8.0. Maximal activity occurred at pH 5.2 to 5.3 and salt concentration had little effect on phosphatase activity up to 1.0 m KCl or NaCl. Progressive loss of enzymatic acitivity occurred at higher salt concentrations. Molecular weight of purified acid phosphatase was estimated to be 58,000.
Full text
PDF

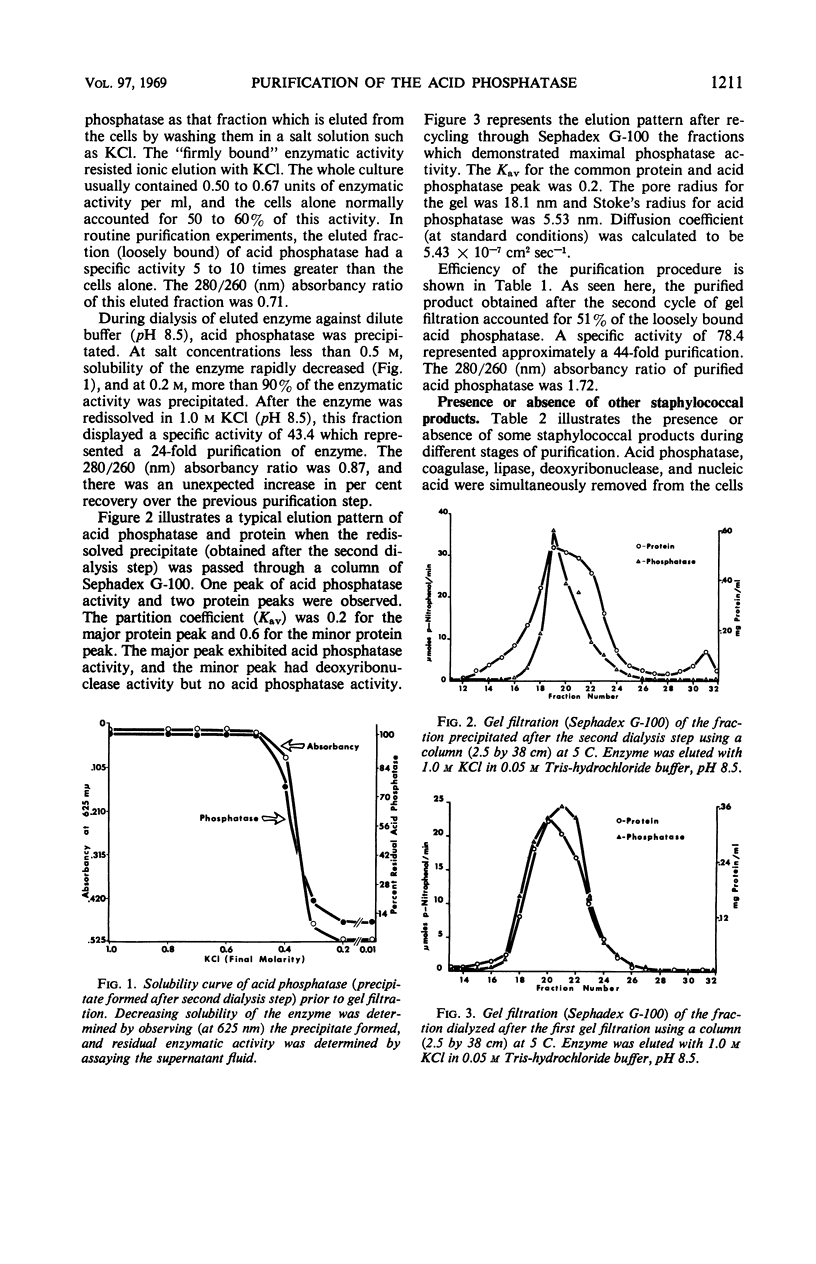
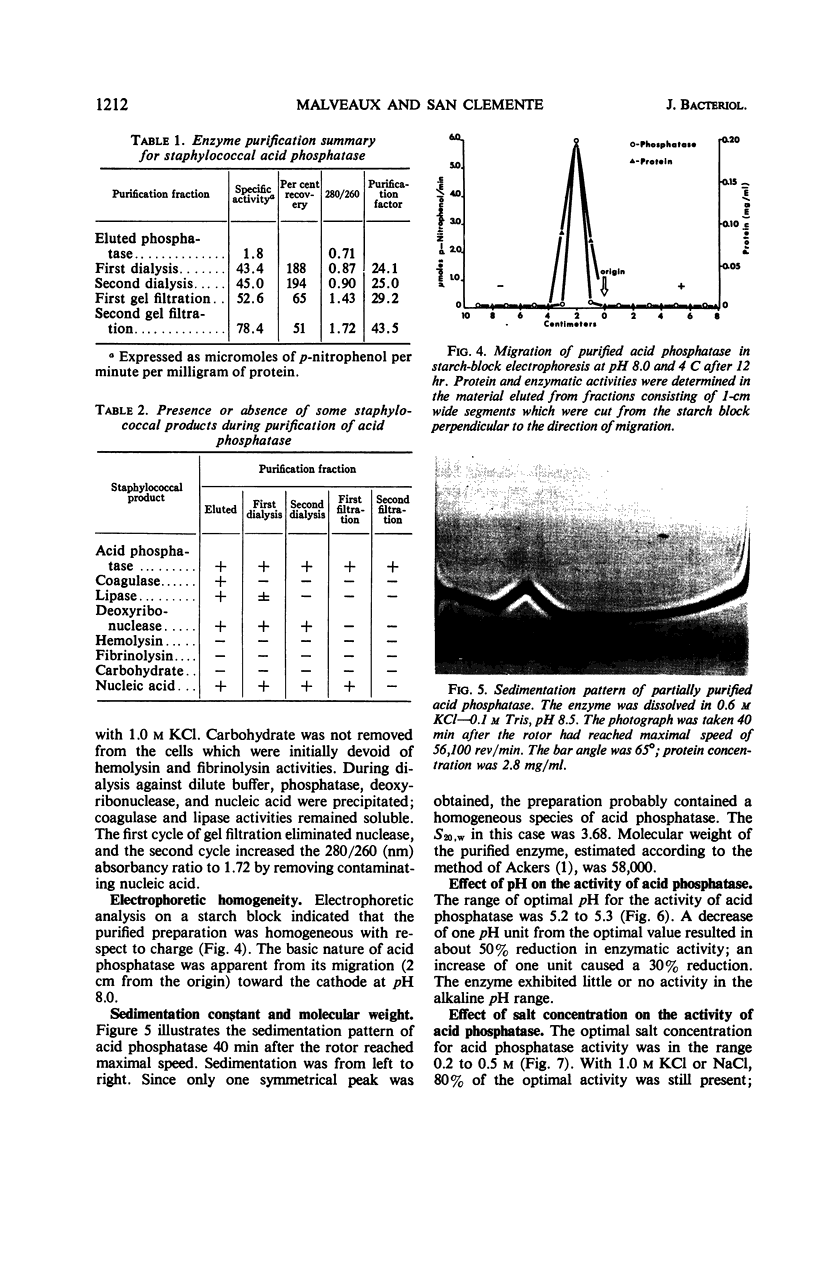
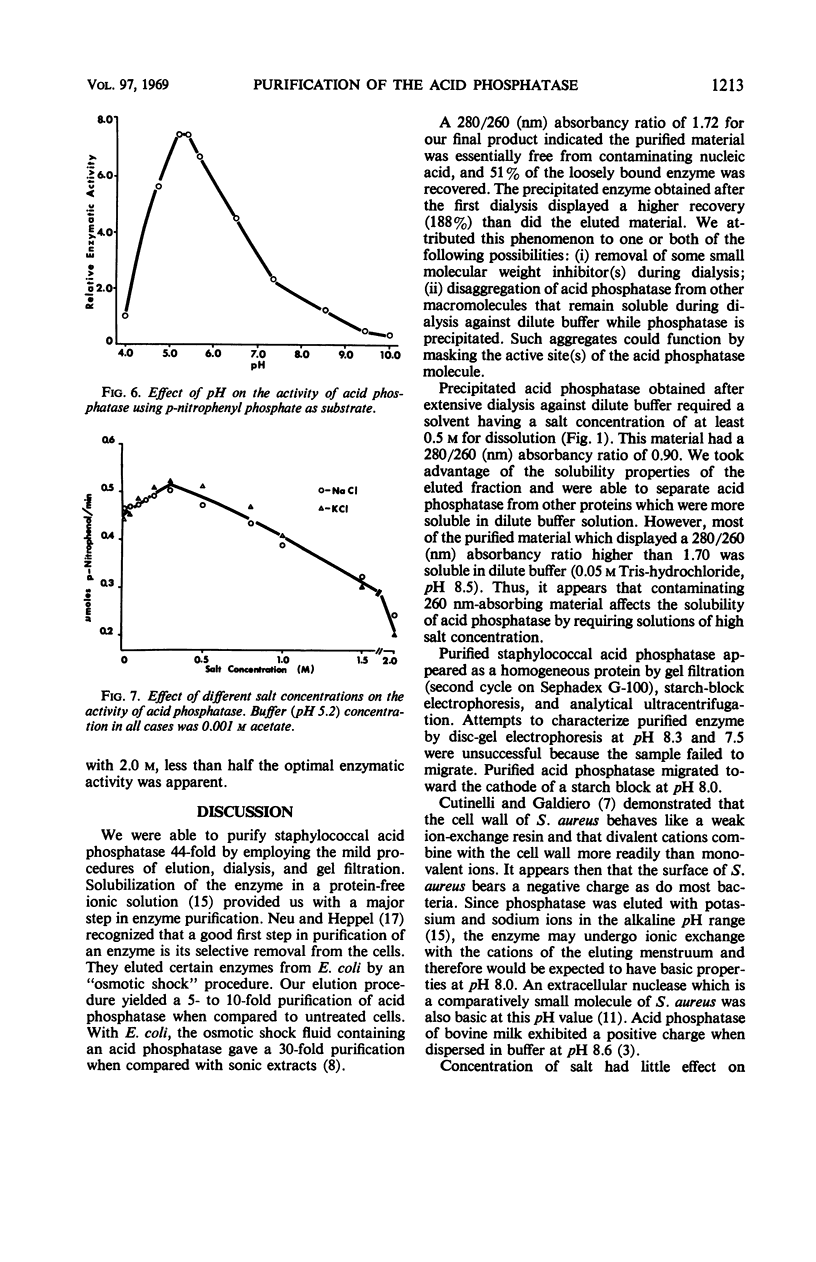

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- BARNES E. H., MORRIS J. F. A quantitative study of the phosphatase activity of Micrococcus pyogenes. J Bacteriol. 1957 Jan;73(1):100–104. doi: 10.1128/jb.73.1.100-104.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BINGHAM E. W., ZITTLE C. A. Purification and properties of acid phosphatase in bovine milk. Arch Biochem Biophys. 1963 Jun;101:471–477. doi: 10.1016/0003-9861(63)90505-8. [DOI] [PubMed] [Google Scholar]
- BLAIR J. E., CARR M. The techniques and interpretation of phage typing of staphylococci. J Lab Clin Med. 1960 Apr;55:650–662. [PubMed] [Google Scholar]
- Cutinelli C., Galdiero F. Ion-binding properties of the cell wall of Staphylococcus aureus. J Bacteriol. 1967 Jun;93(6):2022–2023. doi: 10.1128/jb.93.6.2022-2023.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Brockman R. W., Heppel L. A. Purification and properties of two acid phosphatase fractions isolated from osmotic shock fluid of Escherichia coli. Biochemistry. 1967 Jun;6(6):1743–1751. doi: 10.1021/bi00858a024. [DOI] [PubMed] [Google Scholar]
- FODOR M., ROZGONYI F., CSEPKE E. CORRELATION BETWEEN PHAGE-TYPE, COAGULASE, HYALURONIDASE AND PHOSPHATASE ACTIVITY, AND MERCURIC CHLORIDE RESISTANCE OF STAPHYLOCOCCUS AUREUS. Acta Microbiol Acad Sci Hung. 1963;10:19–25. [PubMed] [Google Scholar]
- Heins J. N., Suriano J. R., Taniuchi H., Anfinsen C. B. Characterization of a nuclease produced by Staphylococcus aureus. J Biol Chem. 1967 Mar 10;242(5):1016–1020. [PubMed] [Google Scholar]
- Kedzia W., Musielak M., Kedzia B., Koniar H., Pniewska E. Enzymatic activity of coagulase-positive Staphylococcus aureus strains isolated from patients and healthy carriers. Pathol Microbiol (Basel) 1966;29(3):307–323. doi: 10.1159/000161915. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malveaux F. J., San Clemente C. L. Elution of loosely bound acid phosphatase from Staphylococcus aureus. Appl Microbiol. 1967 Jul;15(4):738–743. doi: 10.1128/am.15.4.738-743.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., SELIGMAN A. M. Evidence for the specificity of esterase and lipase by the use of three chromogenic substrates. J Biol Chem. 1949 Nov;181(1):343–355. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Shah D. B., Blobel H. Repressible alkaline phosphatase of Staphylococcus aureus. J Bacteriol. 1967 Sep;94(3):780–781. doi: 10.1128/jb.94.3.780-781.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- von HOFSTEN, PORATH J. Purification and some properties of an acid phosphatase from Escherichia coli. Biochim Biophys Acta. 1962 Oct 8;64:1–12. doi: 10.1016/0006-3002(62)90754-0. [DOI] [PubMed] [Google Scholar]