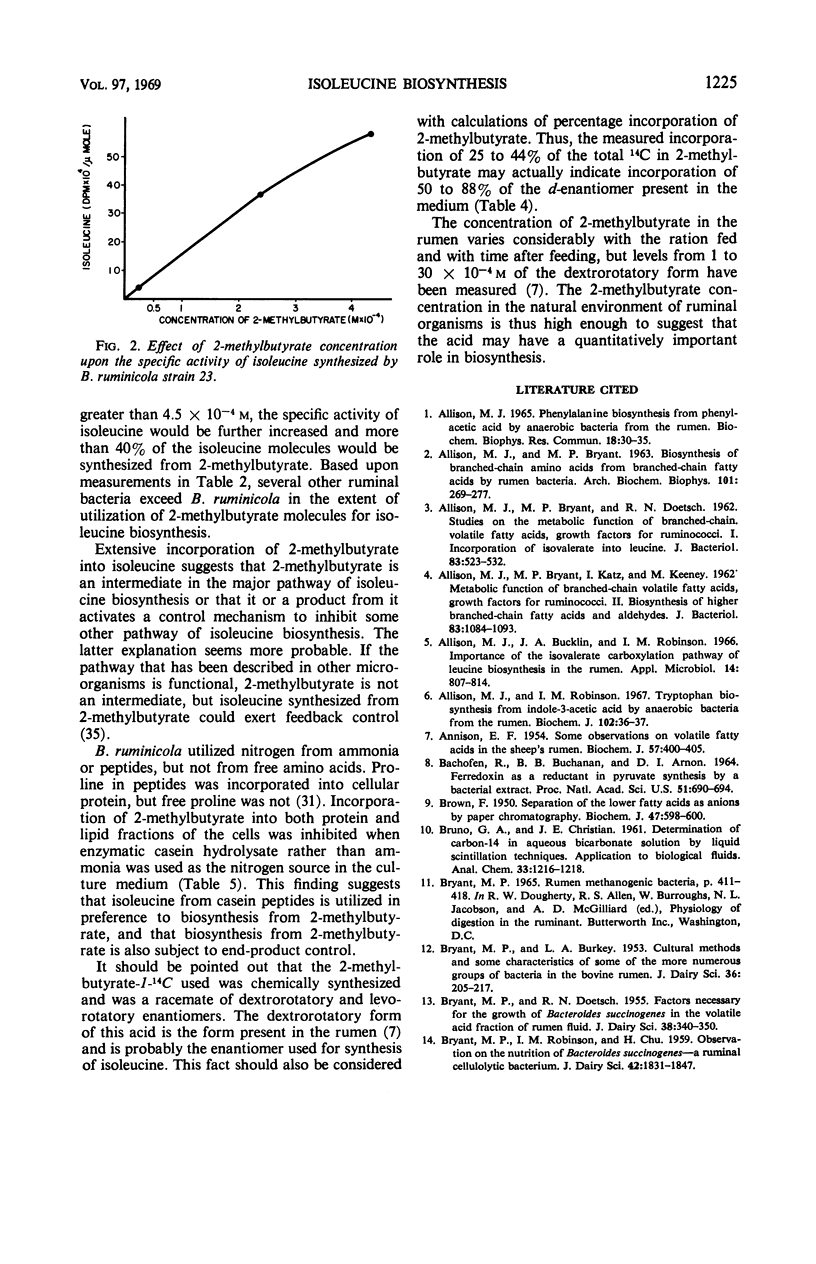
Abstract
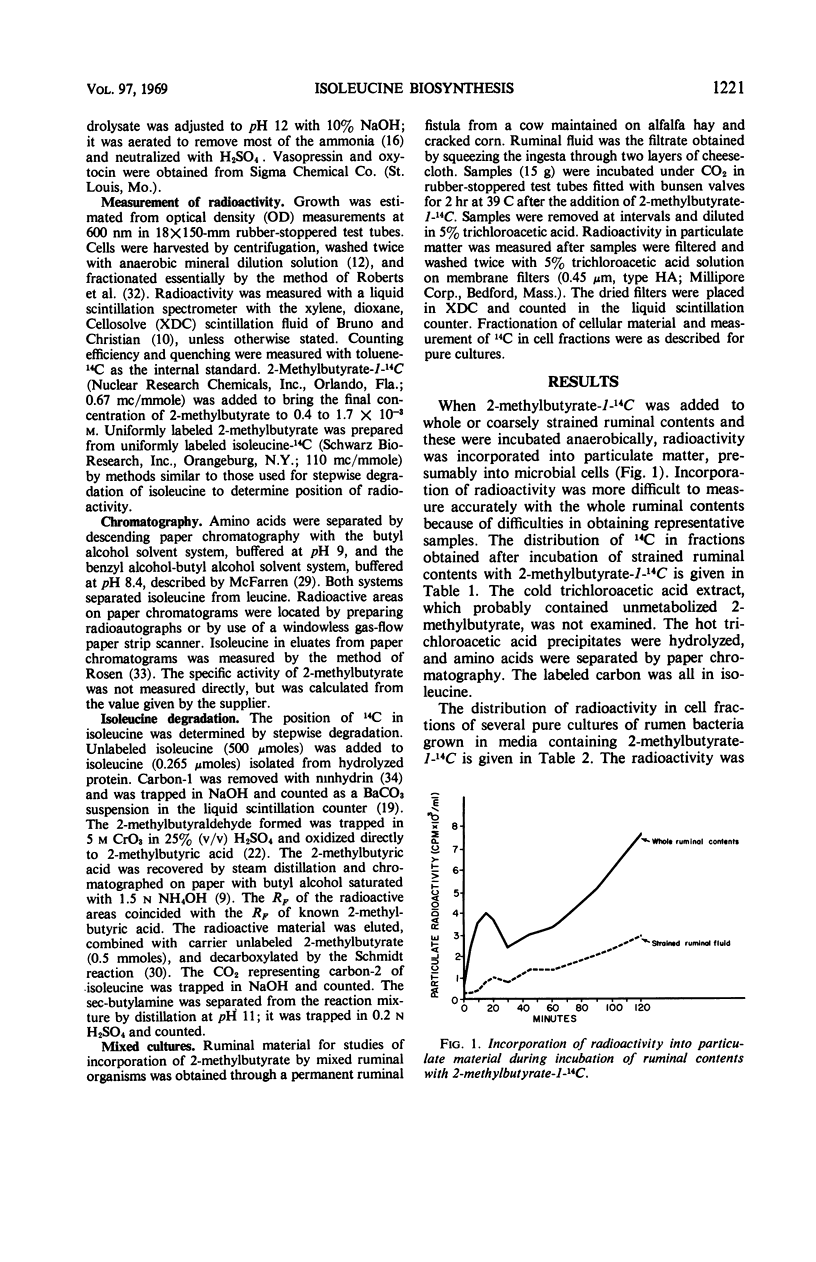
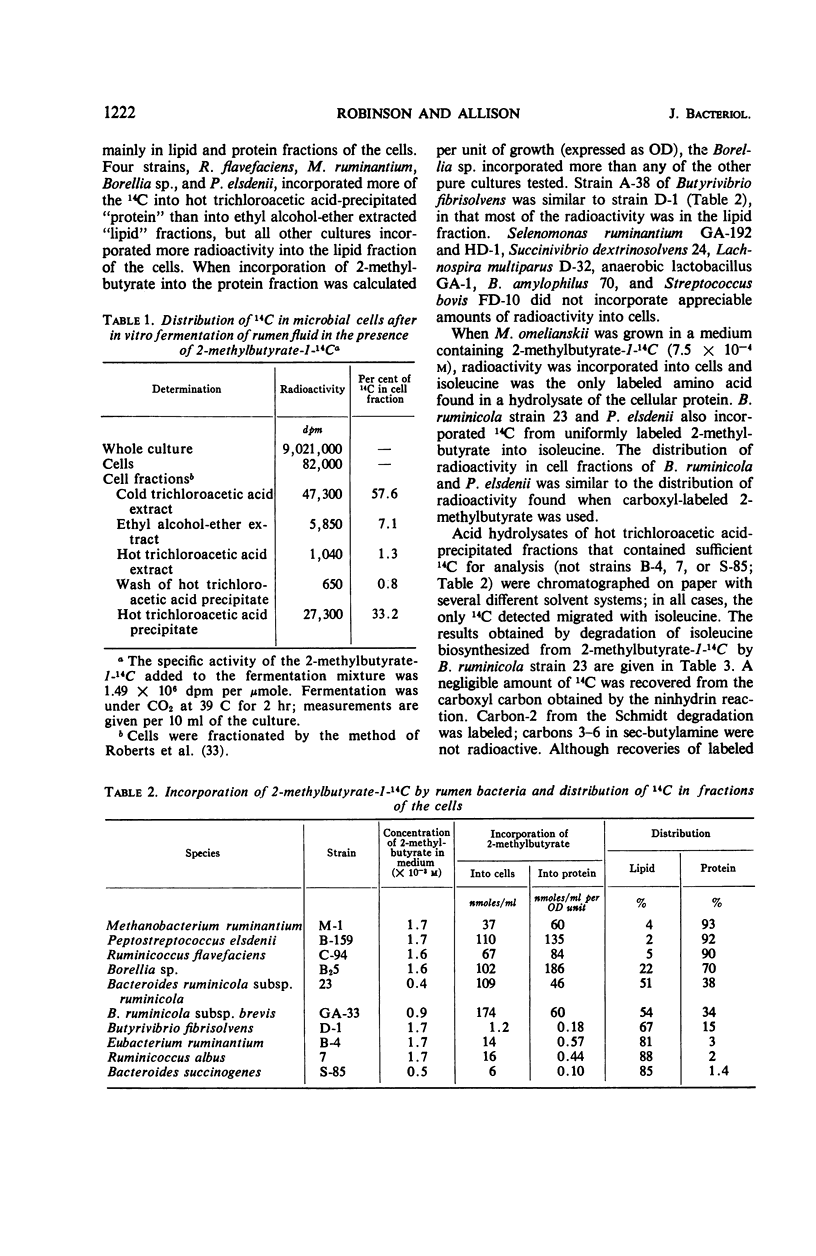
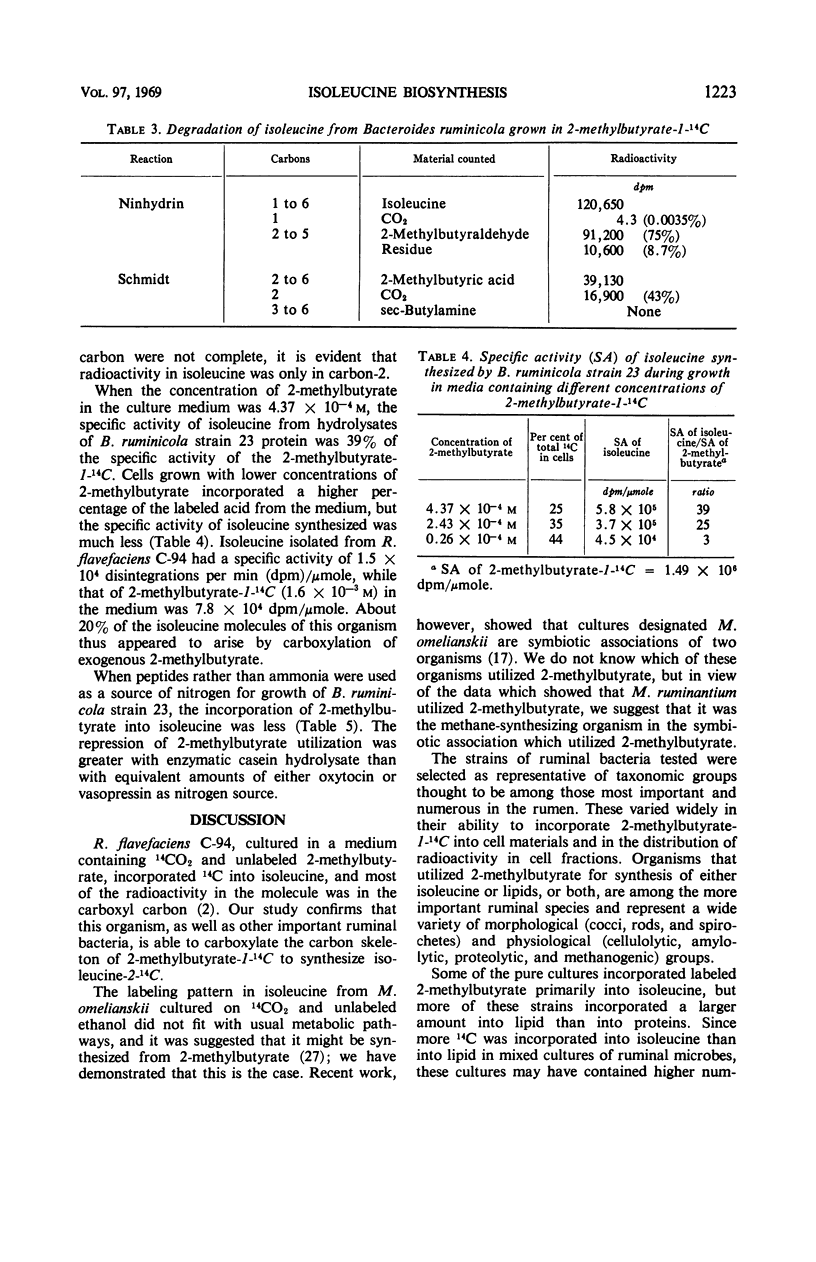
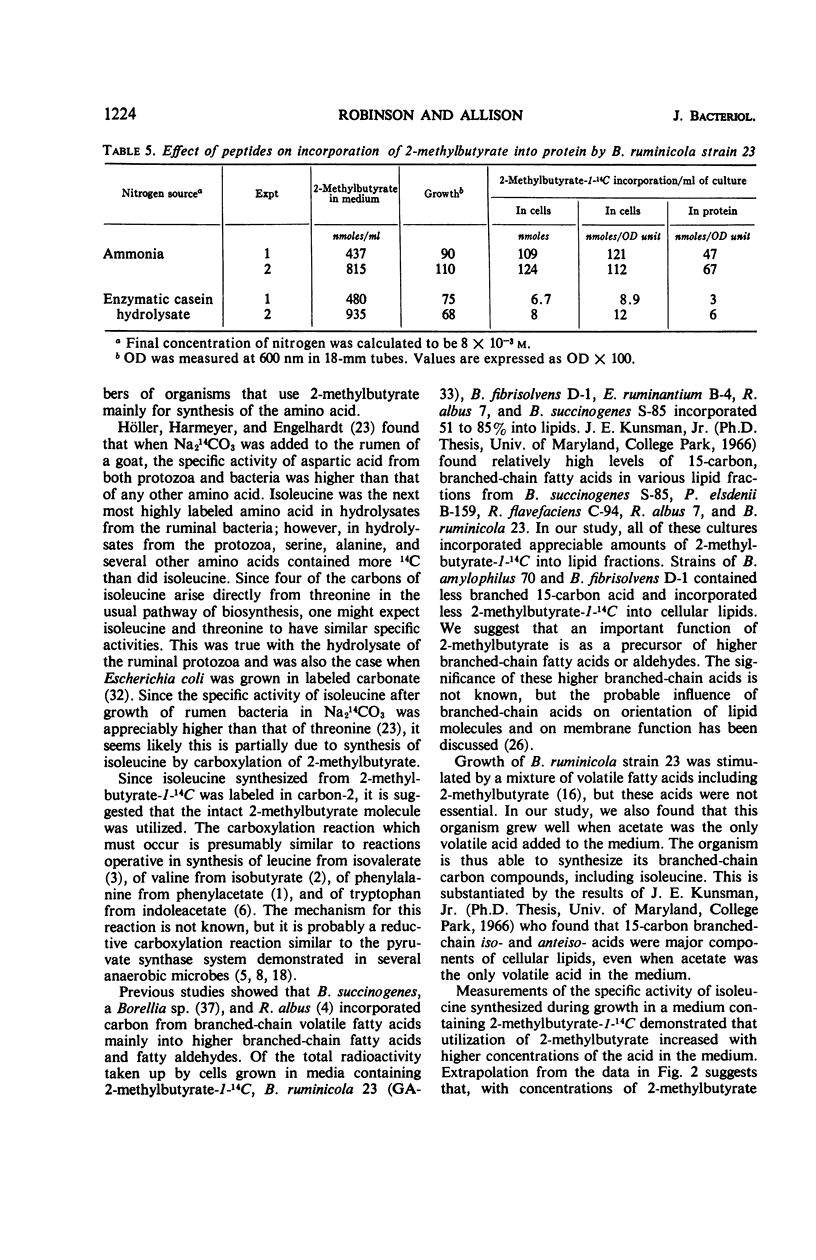
Microorganisms in ruminal ingesta and pure cultures of anaerobic ruminal bacteria of different physiological and morphological groups incorporated 14C from labeled 2-methylbutyrate during growth. The radioactivity was incorporated mainly into lipid and protein. Isoleucine was the only labeled amino acid found in acid hydrolysates of protein from either pure or mixed cultures. Radioactivity in isoleucine synthesized from 2-methylbutyrate-1-14C was entirely in carbon-2. Thus, the carboxylation of 2-methylbutyrate is a pathway for synthesis of isoleucine different from that operative in many aerobic and facultative microorganisms. The specific activity of isoleucine from 2-methylbutyrate by Bacteroides rumminicola 23 increased with higher concentrations of 2-methylbutyrate (2.6 to 44 × 10−5m) in the growth medium. At the highest concentration, the specific activity of isoleucine synthesized was 40% of the specific activity of the 2-methylbutyrate in the growth medium. The use of enzymatic casein hydrolysate, oxytocin, or vasopressin rather than ammonia as nitrogen source for growth of strain 23 depressed the incorporation of 2-methylbutyrate into isoleucine. Synthesis of isoleucine from 2-methylbutyrate appears to be an important reaction in the rumen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P. Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Arch Biochem Biophys. 1963 May;101:269–277. doi: 10.1016/s0003-9861(63)80012-0. [DOI] [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Studies on the metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. I. Incorporation of isovalerate into leucine. J Bacteriol. 1962 Mar;83:523–532. doi: 10.1128/jb.83.3.523-532.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON M. J. PHENYLALANINE BIOSYNTHESIS FROM PHENYLACETIC ACID BY ANAEROBIC BACTERIA FROM THE RUMEN. Biochem Biophys Res Commun. 1965 Jan 4;18:30–35. doi: 10.1016/0006-291x(65)90877-6. [DOI] [PubMed] [Google Scholar]
- ANNISON E. F. Some observations on volatile fatty acids in the sheep's rumen. Biochem J. 1954 Jul;57(3):400–405. doi: 10.1042/bj0570400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Bucklin J. A., Robinson I. M. Importance of the isovalerate carboxylation pathway of leucine biosynthesis in the rumen. Appl Microbiol. 1966 Sep;14(5):807–814. doi: 10.1128/am.14.5.807-814.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHOFEN R., BUCHANAN B. B., ARNON D. I. FERREDOXIN AS A REDUCTANT IN PYRUVATE SYNTHESIS BY A BACTERIAL EXTRACT. Proc Natl Acad Sci U S A. 1964 Apr;51:690–694. doi: 10.1073/pnas.51.4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN F. Separation of the lower fatty acids as anions by paper chromatography. Biochem J. 1950 Nov-Dec;47(5):598–600. doi: 10.1042/bj0470598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHANAN B. B., BACHOFEN R., ARNON D. I. ROLE OF FERREDOXIN IN THE REDUCTIVE ASSIMILATION OF CO2 AND ACETATE BY EXTRACTS OF THE PHOTOSYNTHETIC BACTERIUM, CHROMATIUM. Proc Natl Acad Sci U S A. 1964 Sep;52:839–847. doi: 10.1073/pnas.52.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Dehority B. A. Characterization of several bovine rumen bacteria isolated with a xylan medium. J Bacteriol. 1966 May;91(5):1724–1729. doi: 10.1128/jb.91.5.1724-1729.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Munro C. O. Nutritional requirements of anaerobic spirochetes. I. Demonstration of isobutyrate and bicarbonate as growth factors for a strain of Treponema microdentium. J Bacteriol. 1966 Jan;91(1):27–32. doi: 10.1128/jb.91.1.27-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. S., Gibson J. Photoassimilation of acetate and the biosynthesis of amino acids by Chlorobium thiosulphatophilum. Biochem J. 1964 Jun;91(3):546–559. doi: 10.1042/bj0910546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Wolfe R. S., Elsden S. R. The synthesis of amino acids by Methanobacterium omelianskii. Biochem J. 1966 Apr;99(1):76–86. doi: 10.1042/bj0990076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSON A. D., HATTIER L. V., MCCLESKEY C. S. VOLATILE FATTY ACID REQUIREMENT OF A STRAIN OF LISTERIA MONOCYTOGENES. J Bacteriol. 1965 Mar;89:819–824. doi: 10.1128/jb.89.3.819-824.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHARES E. F. Degradation of labeled propionic and acetic acids. Arch Biochem Biophys. 1951 Sep;33(2):173–178. doi: 10.1016/0003-9861(51)90094-x. [DOI] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- WEGNER G. H., FOSTER E. M. Incorporation of isobutyrate and valerate into cellular plasmalogen by Bacteroides succinogenes. J Bacteriol. 1963 Jan;85:53–61. doi: 10.1128/jb.85.1.53-61.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


