Abstract
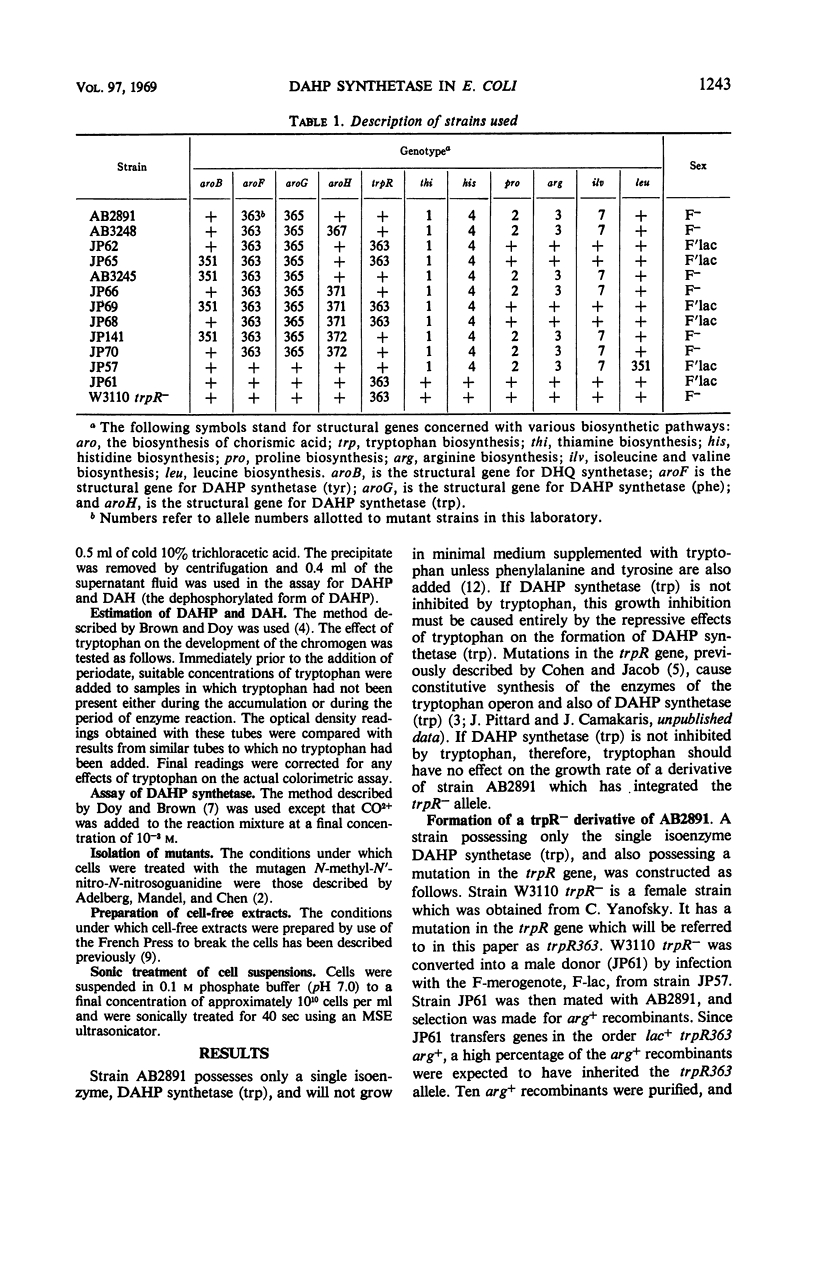
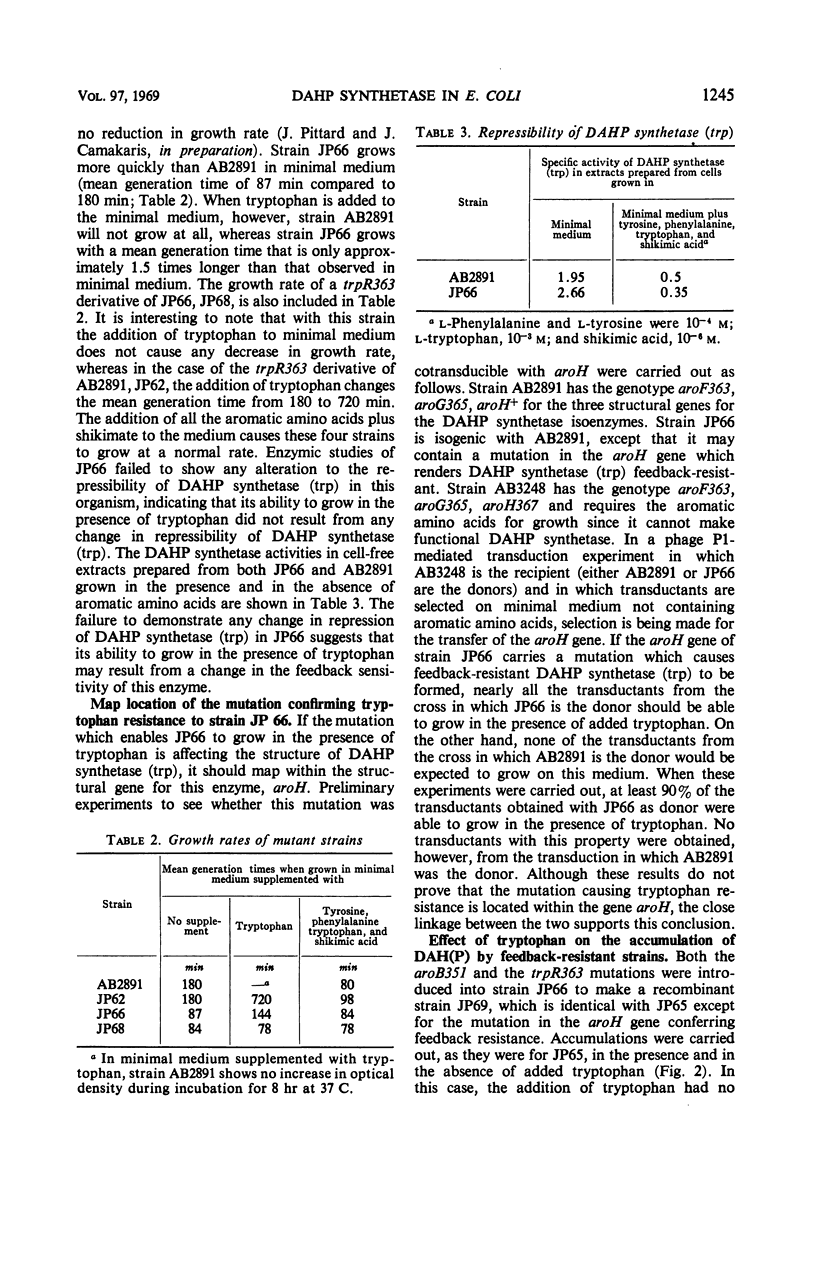
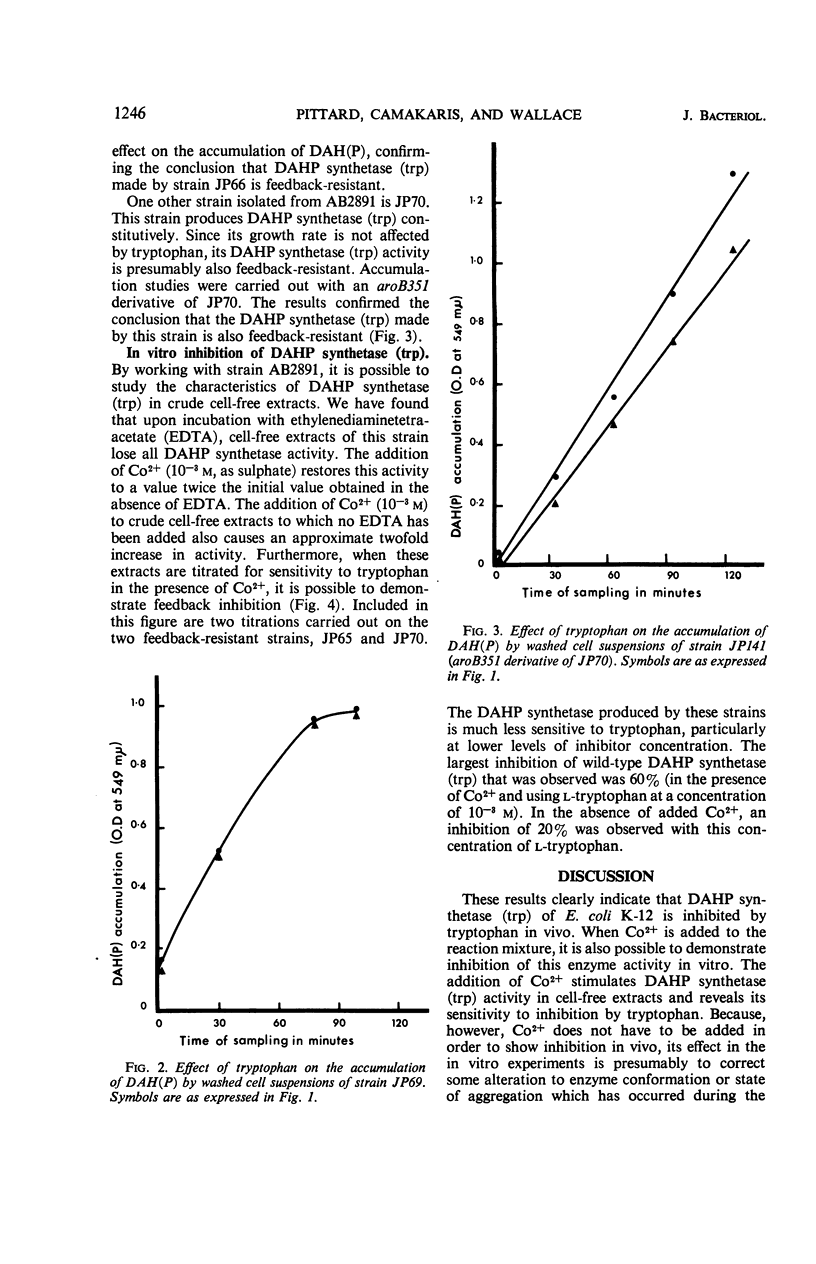
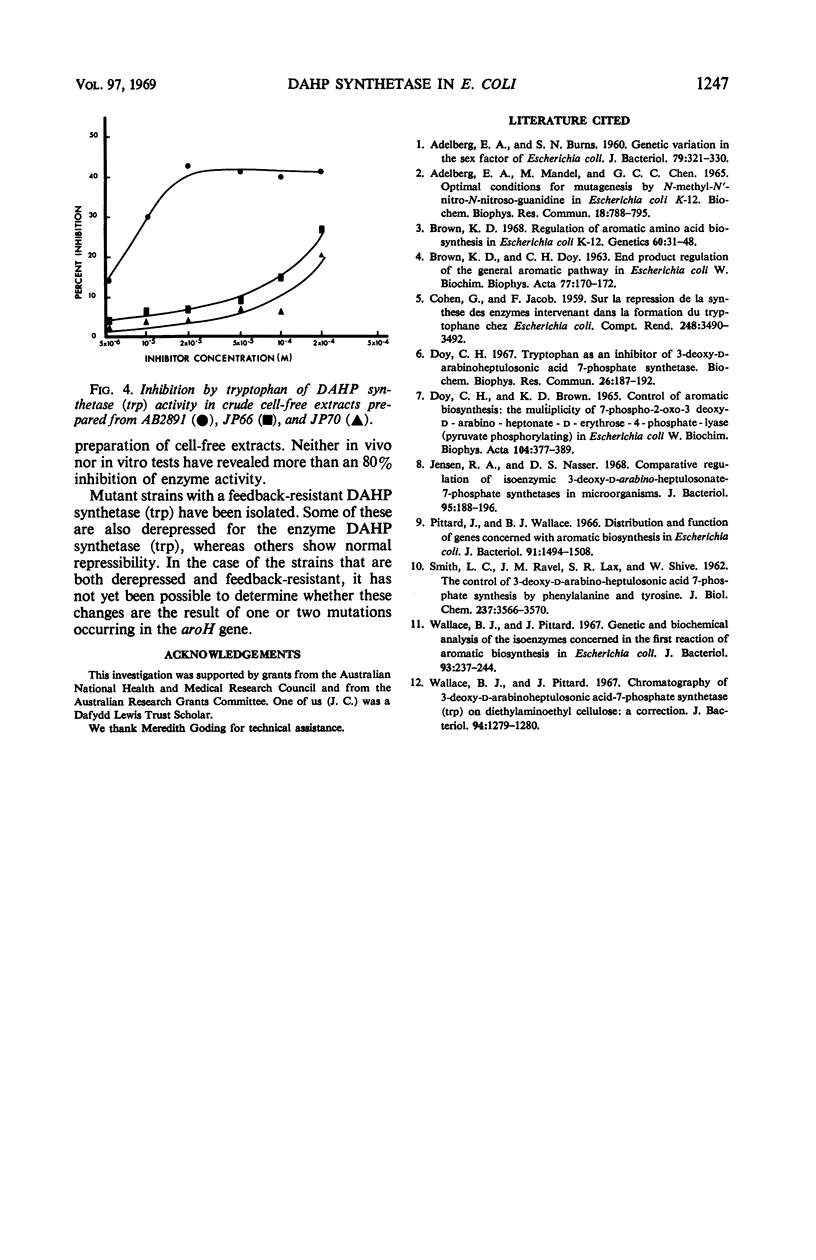
The growth of a strain of Escherichia coli K-12 which possesses only the single isoenzyme 3-deoxy-d-arabinoheptulosonic acid-7-phosphate (DAHP) synthetase (trp), and which makes this enzyme constitutively, is inhibited by tryptophan. The accumulation of DAHP by a derivative of this strain unable to convert DAHP to dehydroquinate is also inhibited by tryptophan. The enzymic activity of DAHP synthetase (trp) in the presence of CO2+ is sensitive to inhibition by tryptophan. Mutant strains of E. coli have been isolated in which DAHP synthetase (trp) is feedback-resistant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN K. D., DOY C. H. END-PRODUCT REGULATION OF THE GENERAL AROMATIC-PATHWAY IN ESCHERICHIA COLI W. Biochim Biophys Acta. 1963 Sep 3;77:170–172. doi: 10.1016/0006-3002(63)90489-x. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- Doy C. H., Brown K. D. Control of aromatic biosynthesis: the multiplicity of 7-phospho-2-oxo-3-deoxy-D-arabino-heptonate D-erythrose-4-phosphate-lyase (pyruvate-phosphorylating) in Escherichia coli W. Biochim Biophys Acta. 1965 Jul 8;104(2):377–389. doi: 10.1016/0304-4165(65)90343-0. [DOI] [PubMed] [Google Scholar]
- Doy C. H. Tryptophan as an inhibitor of 3-deoxy-arabino-heptulosonate 7-phosphate synthetase. Biochem Biophys Res Commun. 1967 Jan 23;26(2):187–192. doi: 10.1016/0006-291x(67)90232-x. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S. Comparative regulation of isoenzymic 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetases in microorganisms. J Bacteriol. 1968 Jan;95(1):188–196. doi: 10.1128/jb.95.1.188-196.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. The control of 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthesis by phenylalanine and tyrosine. J Biol Chem. 1962 Nov;237:3566–3570. [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Chromatography of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) on diethylaminoethyl cellulose: a correction. J Bacteriol. 1967 Oct;94(4):1279–1280. doi: 10.1128/jb.94.4.1279-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



