Abstract
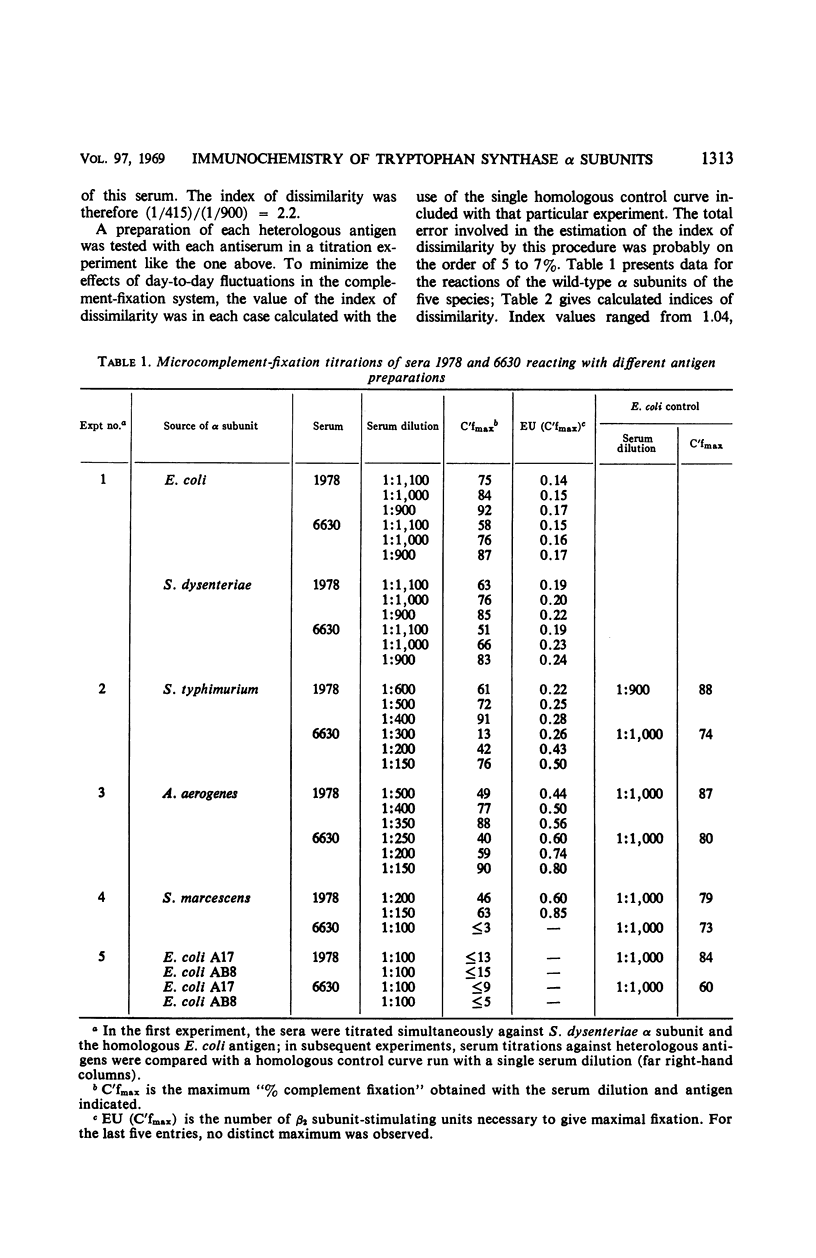
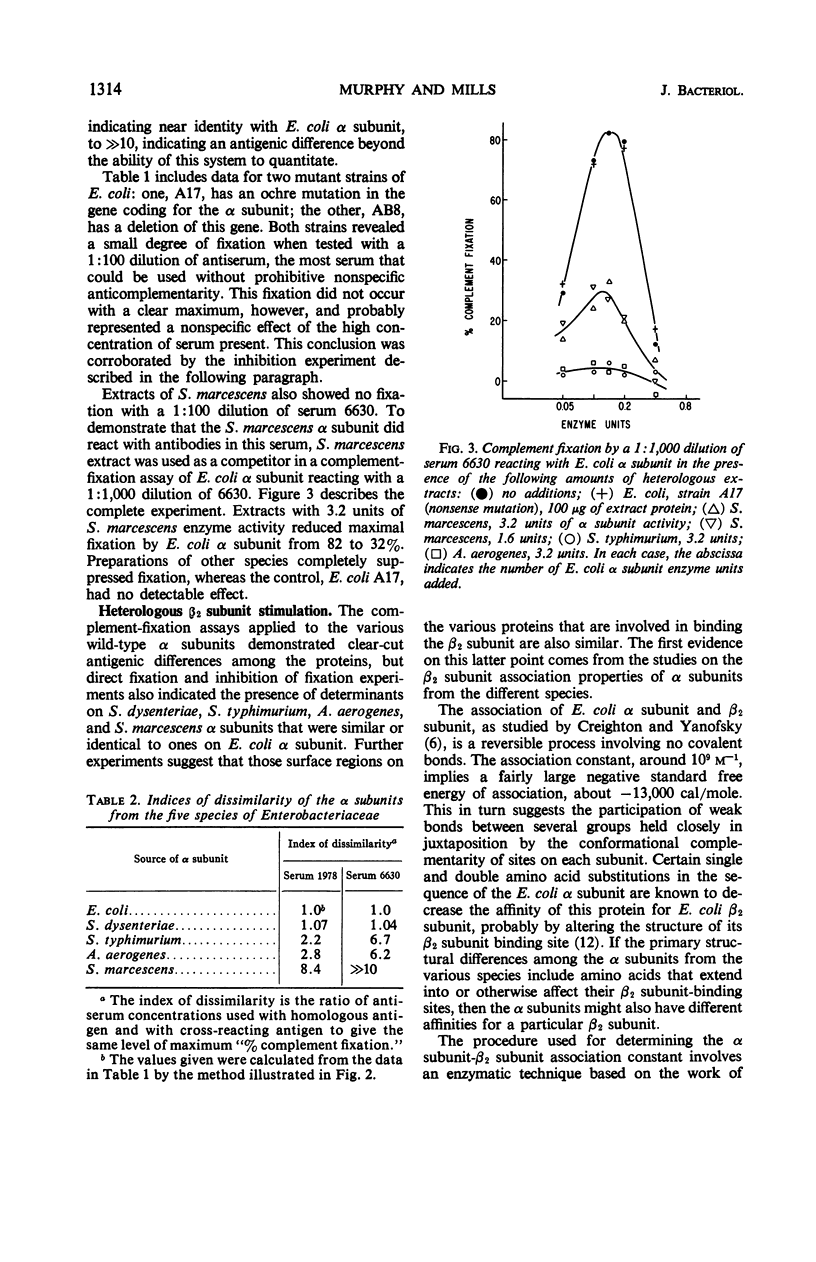
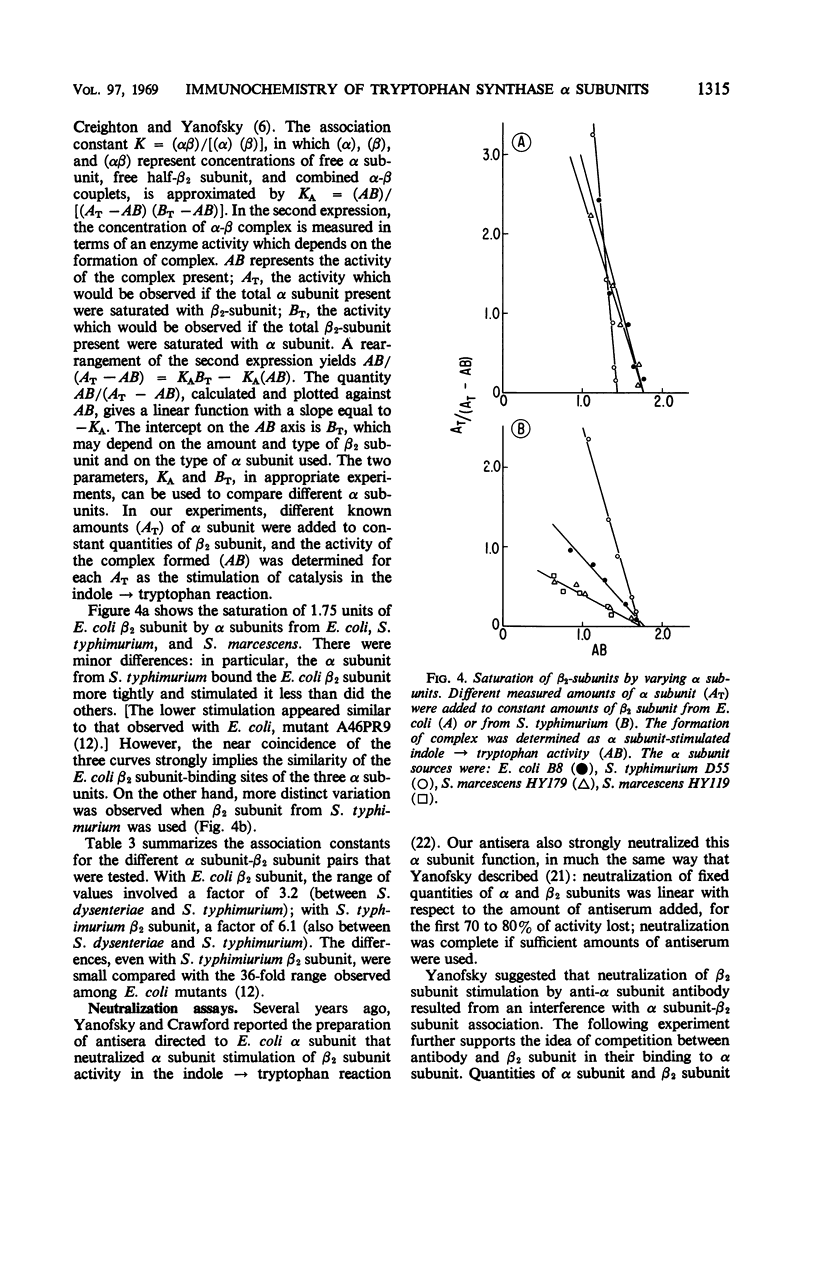
The reactive surface structures of α subunits of tryptophan synthase from Escherichia coli, Shigella dysenteriae, Salmonella typhimurium, Aerobacter aerogenes, and Serratia marcescens were compared by measuring (i) their reactivities in micro-complement-fixation assays with antibodies directed specifically to E. coli wild-type α subunit, (ii) their reactivities in enzyme neutralization assays with the same antibodies, and (iii) their binding affinities for tryptophan synthase β2 subunits. The enzymes from the four heterologous species cross-reacted in the microcomplement-fixation assays with the anti-E. coli α subunit antibodies, each to a different degree. However, neutralization titers of the antibodies reacting with the various α subunits were comparatively similar, and the β2 subunit-binding and -stimulating abilities of the α subunits were even more closely alike. The results suggested that the tertiary structure of the β2 subunit-binding site of the α subunit has been conserved, relative to the rest of the molecule, during the evolutionary divergence of the species of Enterobacteriaceae.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A. J., Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. doi: 10.1093/genetics/53.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD I. P. Identification of the triose phosphate formed in the tryptophan synthetase reaction. Biochim Biophys Acta. 1960 Dec 4;45:405–407. doi: 10.1016/0006-3002(60)91474-8. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Helinski D. R., Somerville R. L., Yanofsky C. Comparison of the tryptophan synthetase alpha-subunits of several species of Enterobacteriaceae. J Bacteriol. 1966 May;91(5):1819–1826. doi: 10.1128/jb.91.5.1819-1826.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- Freedman M. H., Slobin L. I., Robbins J. B., Sela M. Soluble antigen-antibody complexes as intermediates in the purification of antibodies in 8 molar urea. Arch Biochem Biophys. 1966 Sep 26;116(1):82–91. doi: 10.1016/0003-9861(66)90015-4. [DOI] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical comparisons of mutant and wild-type alpha-subunits of tryptophan synthetase. Arch Biochem Biophys. 1968 Sep 20;127(1):7–16. doi: 10.1016/0003-9861(68)90194-x. [DOI] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Quantitative immunochemistry and the evolution of primate albumins: micro-complement fixation. Science. 1966 Dec 23;154(3756):1563–1566. doi: 10.1126/science.154.3756.1563. [DOI] [PubMed] [Google Scholar]
- TASHJIAN A. H., Jr, LEVINE L., MUNSON P. L. IMMUNOASSAY OF PARATHYROID HORMONE BY QUANTITATIVE COMPLEMENT FIXATION. Endocrinology. 1964 Feb;74:244–254. doi: 10.1210/endo-74-2-244. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- YANOFSKY C. A second reaction catalyzed by the tryptophan synthetase of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):408–416. doi: 10.1016/0006-3002(59)90015-0. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. Antibodies to the two protein components of Escherichia coli tryptophan synthetase. Ann N Y Acad Sci. 1963 May 8;103:1067–1074. doi: 10.1111/j.1749-6632.1963.tb53758.x. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Crawford I. P. THE EFFECTS OF DELETIONS, POINT MUTATIONS, REVERSIONS AND SUPPRESSOR MUTATIONS ON THE TWO COMPONENTS OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Jul;45(7):1016–1026. doi: 10.1073/pnas.45.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]


