Abstract
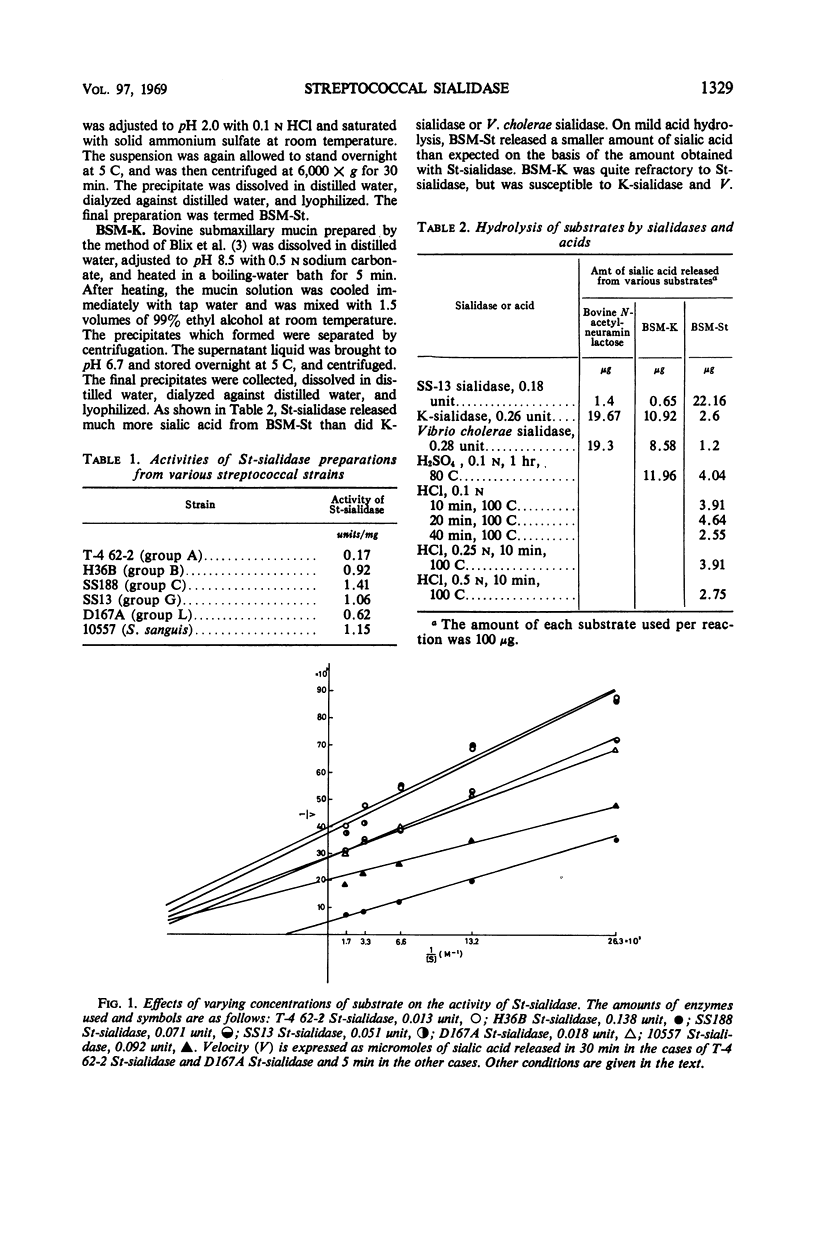
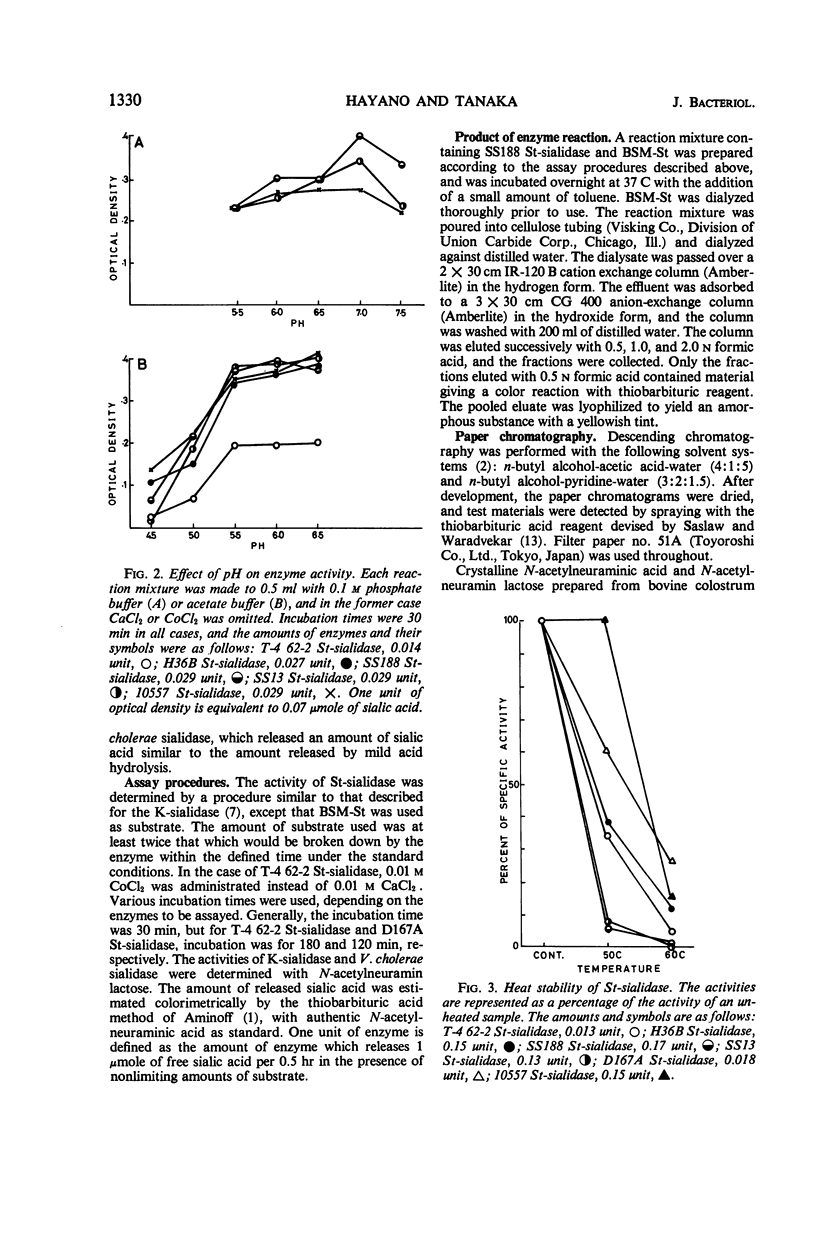
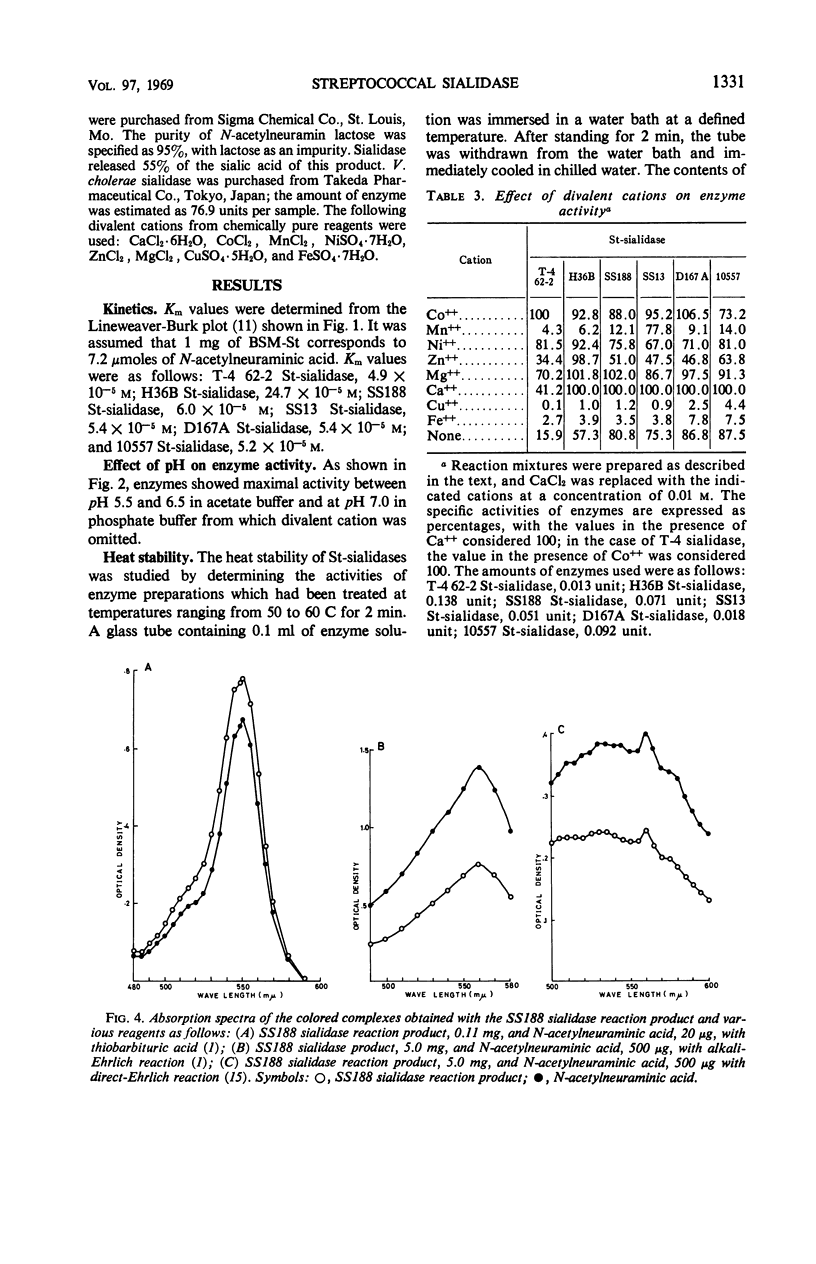
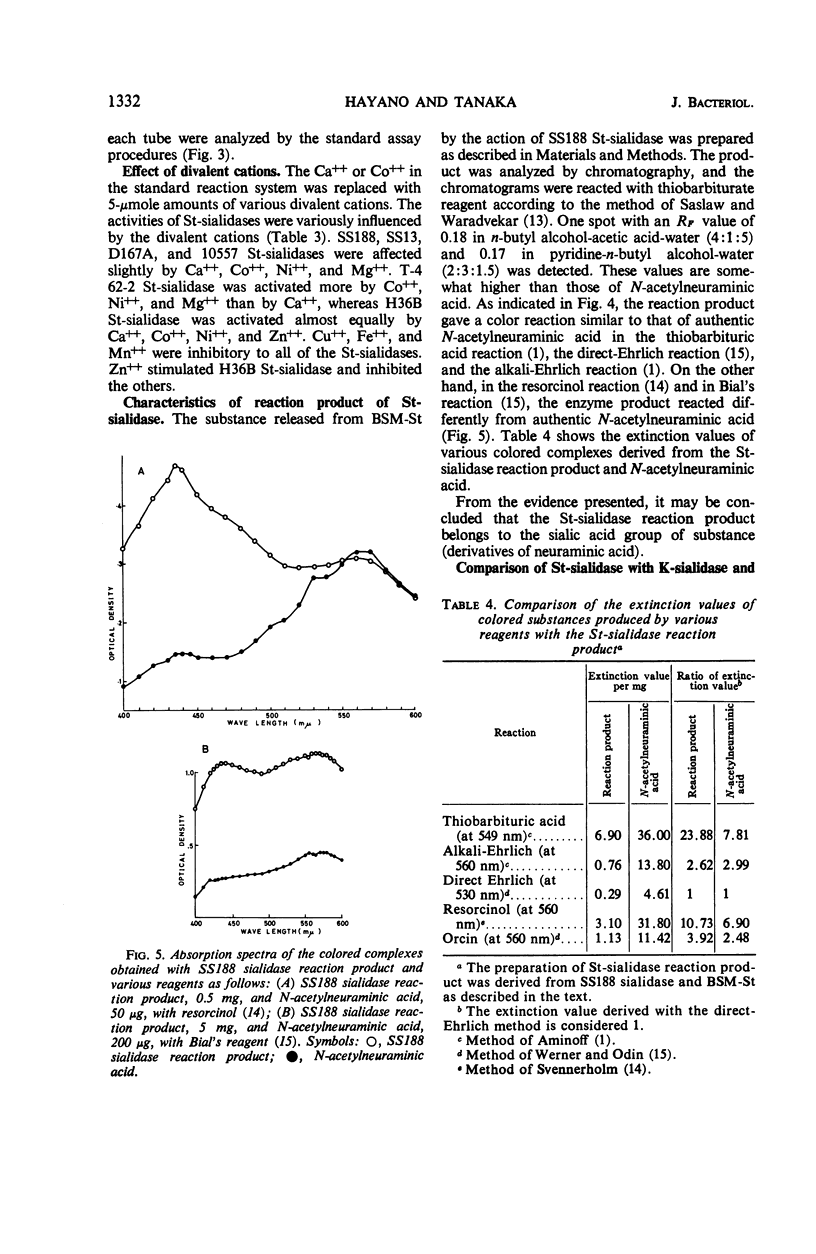
A group of enzymes were prepared from the culture fluids of streptococci belonging to groups A, B, C, G, and L, and from a strain of Streptococcus sanguis. These streptococcal enzymes (designated St-sialidases) released a substance shown to belong to the sialic acid group from the specific substrate BSM-St, a sialomucoid prepared from bovine submaxillary gland. They were inactive on N-acetylneuramin lactose prepared from bovine colustrum and on a sialomucoid prepared from bovine submaxillary mucin, whereas these substances are susceptible to sialidases produced by group K streptococci and by Vibrio cholerae. Some of the St-sialidases were markedly activated by divalent cations, but others showed little response. The heat stability of the enzymes produced by the different strains varied. The optimal pH was between 5.5 and 6.5 with acetate buffer and was about 7 with phosphate buffer. Km values were determined for the St-sialidases with BSM-St as substrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIX G., LINDBERG E., ODIN L., WERNER I. Studies on sialic acids. Acta Soc Med Ups. 1956 Jun 30;61(1-2):1–25. [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Drzeniek R. Differences in splitting capacity of virus and V. cholerae neuraminidases on sialic acid type substrates. Biochem Biophys Res Commun. 1967 Mar 21;26(6):631–638. doi: 10.1016/s0006-291x(67)80118-9. [DOI] [PubMed] [Google Scholar]
- Hayano S., Tanaka A. Streptococcal sialidase. I. Isolation and properties of sialidase produced by group K Streptococcus. J Bacteriol. 1967 Jun;93(6):1753–1757. doi: 10.1128/jb.93.6.1753-1757.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano S., Tanaka A. Streptococcal sialidase. II. Kinetic and immunological studies of sialidase produced by group K streptococcus. J Bacteriol. 1968 May;95(5):1551–1554. doi: 10.1128/jb.95.5.1551-1554.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacson P. Myxoviruses and autoimmunity. Prog Allergy. 1967;10:256–292. [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Bahn A. N. Extracellular streptococcal neuraminidase. J Bacteriol. 1968 Apr;95(4):1491–1492. doi: 10.1128/jb.95.4.1491-1492.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASLAW L. D., WARAVDEKAR V. S. Detection of some deoxy sugars and aldehydes using thiobarbituric acid. Arch Biochem Biophys. 1960 Oct;90:245–249. doi: 10.1016/0003-9861(60)90575-0. [DOI] [PubMed] [Google Scholar]
- WERNER I., ODIN L. On the presence of sialic acid in certain glycoproteins and in gangliosides. Acta Soc Med Ups. 1952;57(3-4):230–241. [PubMed] [Google Scholar]
- Wilson V. W., Jr, Rafelson M. R., Jr Studies on the neuraminidases of influenza virus. 3. Stimulation of activity by bivalent cations. Biochim Biophys Acta. 1967 Sep 12;146(1):160–166. doi: 10.1016/0005-2744(67)90082-4. [DOI] [PubMed] [Google Scholar]


