Abstract
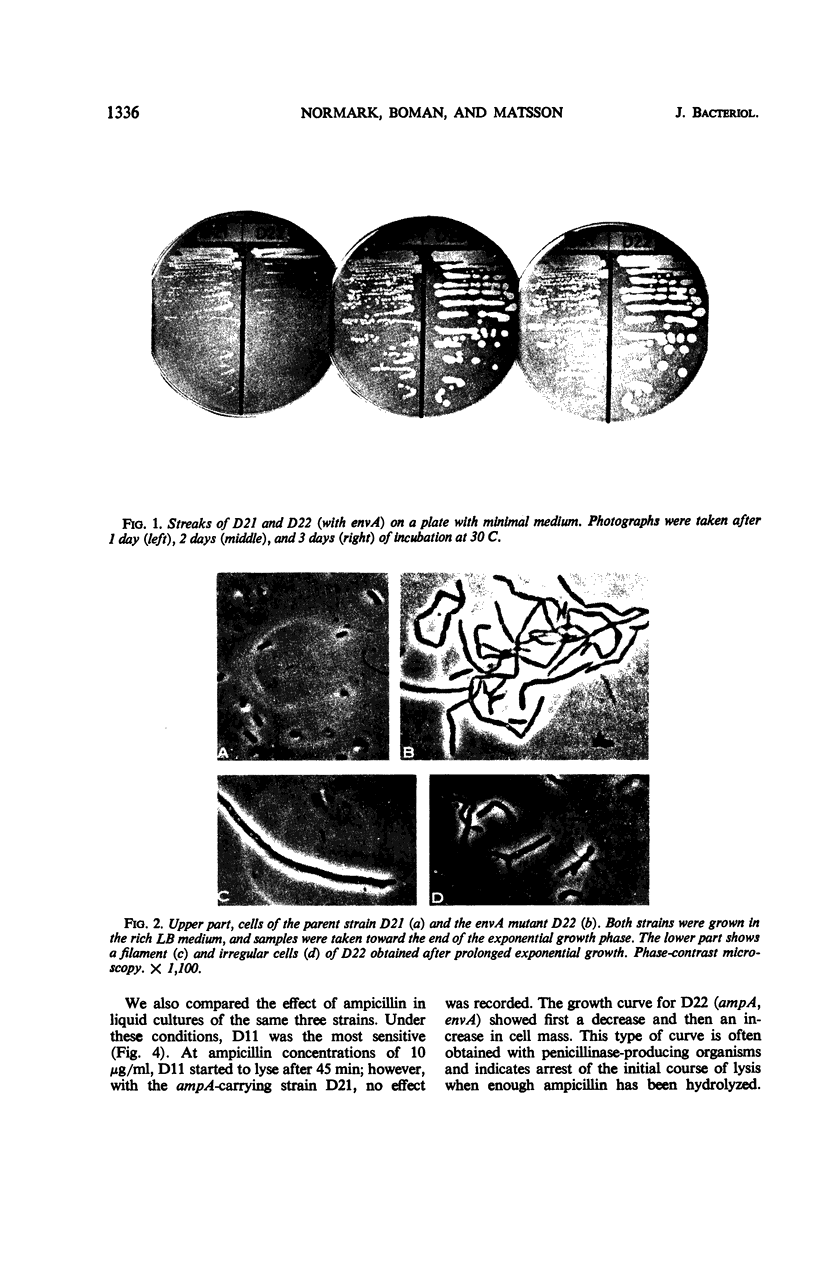
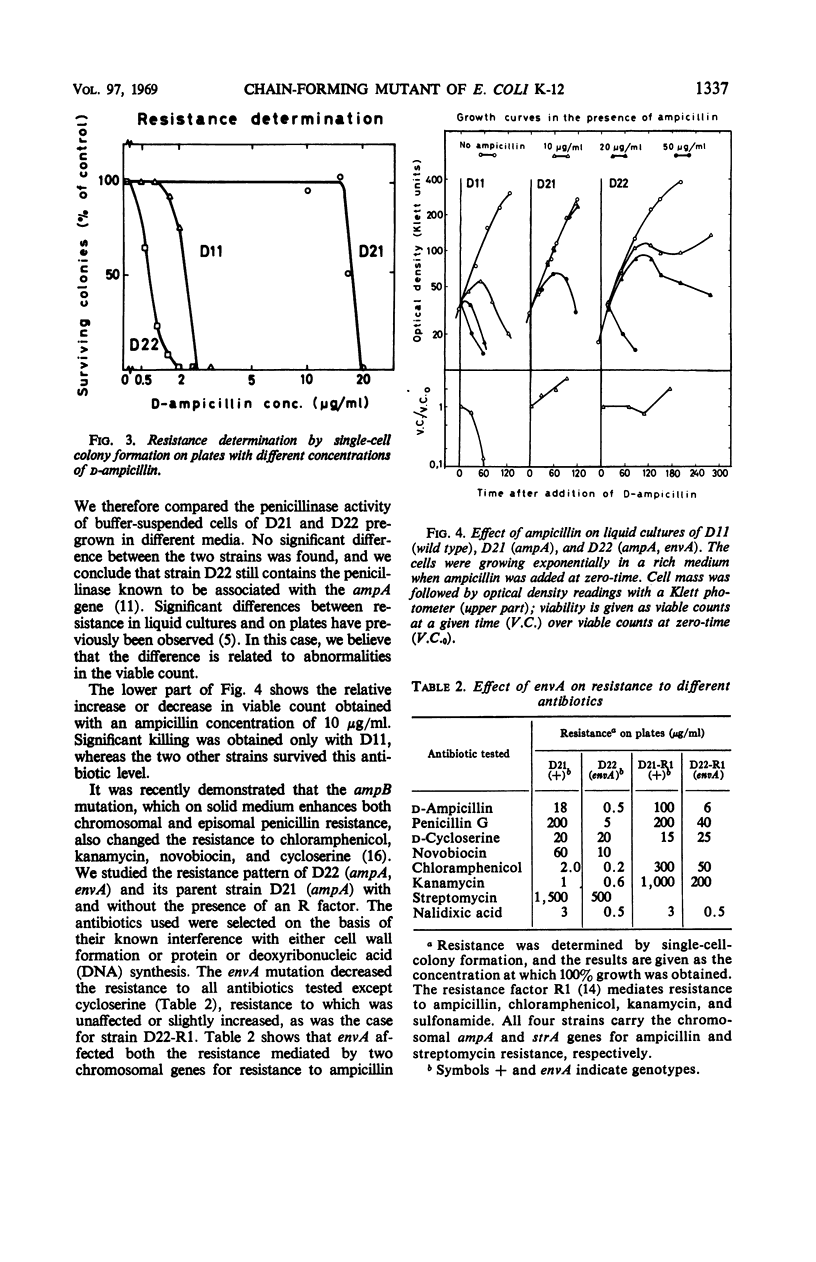
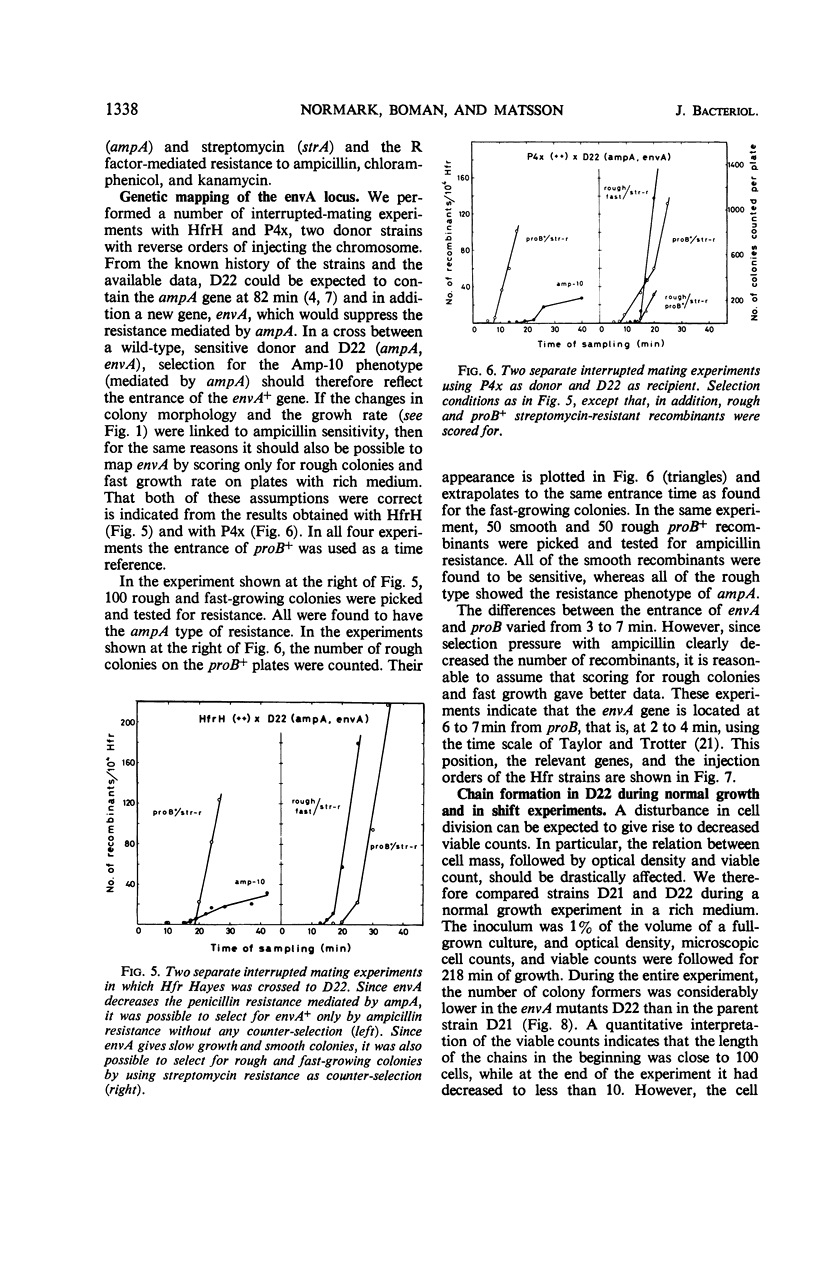
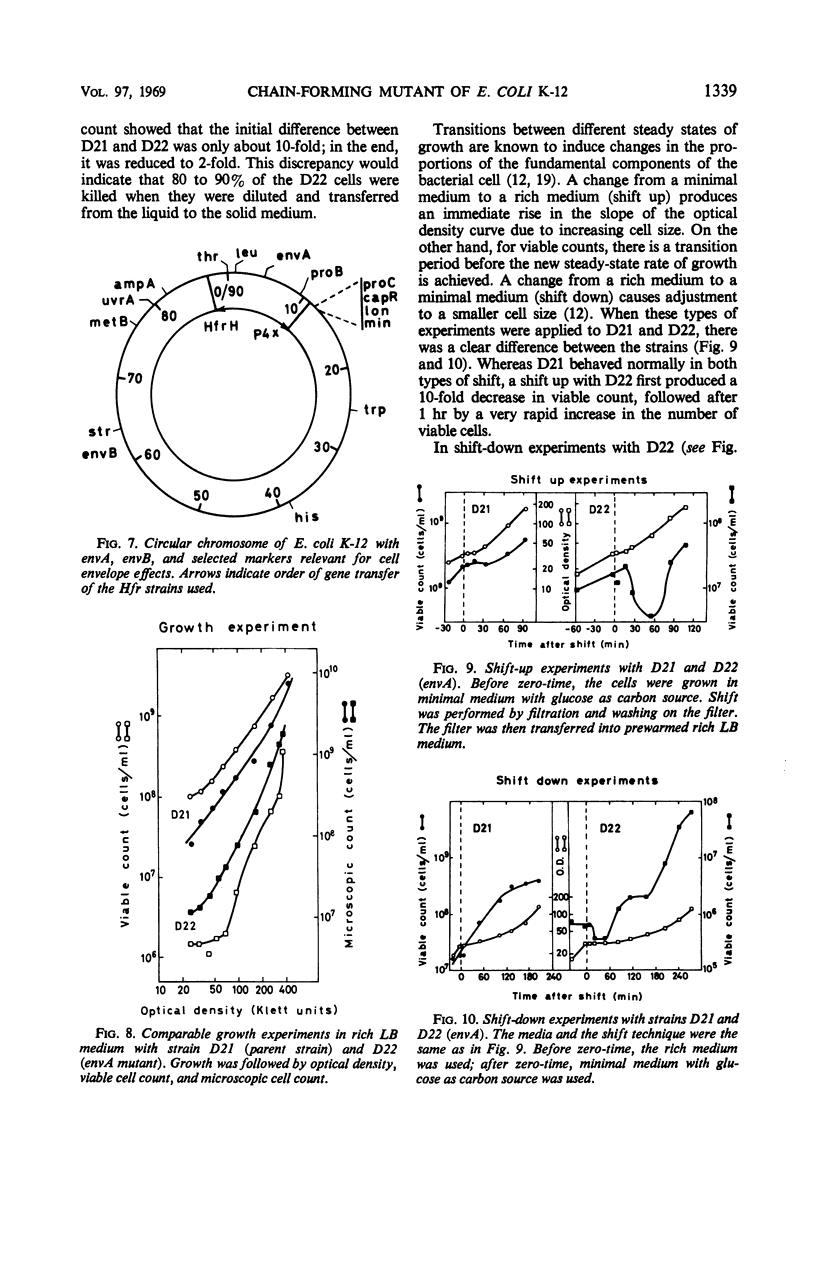
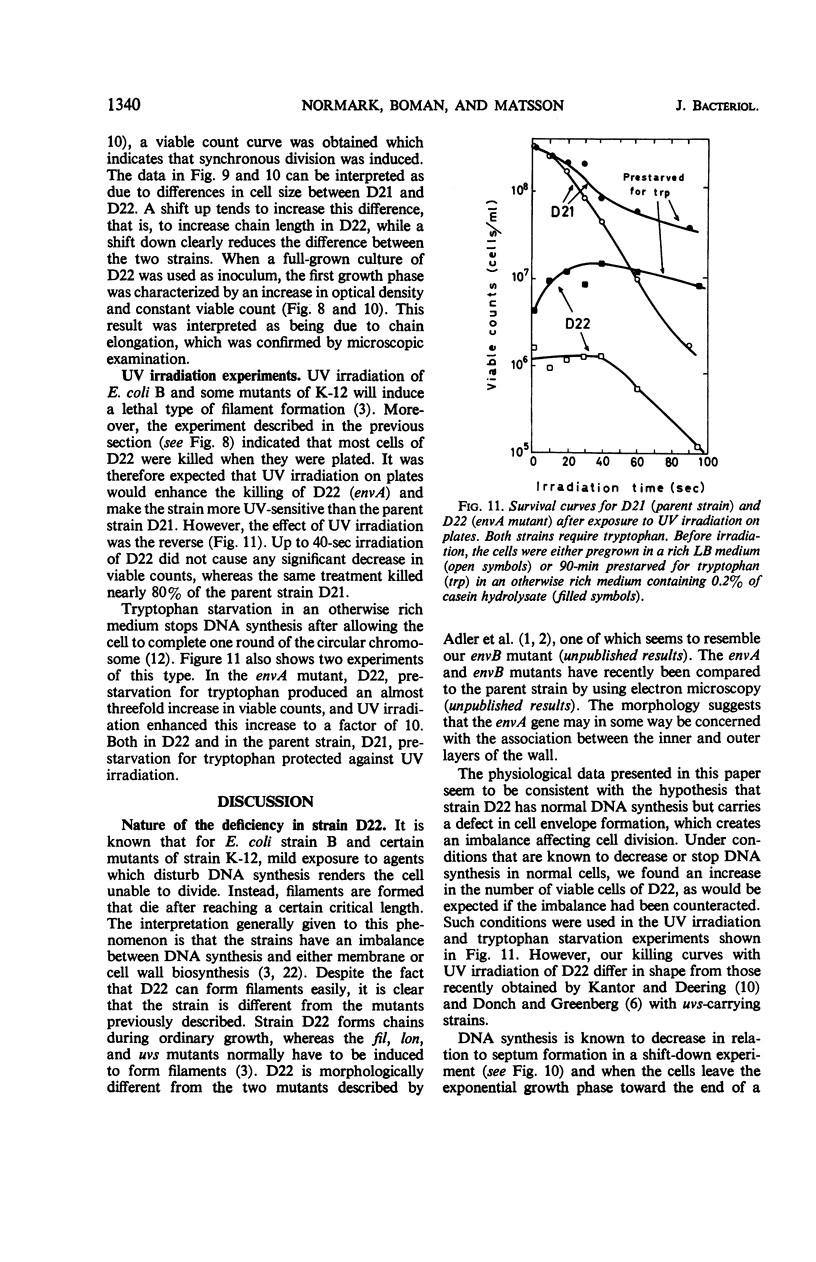
In a mutation experiment with a rough, ampicillin-resistant strain, we isolated two smooth mutants which were both sensitive to ampicillin and carried defects in the cell envelope. One of the strains (with the envA gene) is hindered in its completion of septa and forms chains of cells. The envA gene has been mapped to a position between leu and proB, at 2 to 4 min. The envA gene decreased the resistance mediated by both episomal and chromosomal genes for resistance to several antibiotics. During growth the envA mutant was characterized by abnormal ratios between viable count or cell count and optical density. The ratio between viable count and optical density was affected during shift-up and shift-down experiments. When compared to the parent strain, the envA mutant was found to be more resistant to ultraviolet irradiation on plates. Prestarvation for tryptophan had a protective effect against irradiation both on the parent strain and the envA mutant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Terry C. E., Hardigree A. A. Giant cells of Escherichia coli. J Bacteriol. 1968 Jan;95(1):139–142. doi: 10.1128/jb.95.1.139-142.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazill G. W. Lethal unbalanced growth in bacteria. Nature. 1967 Oct 28;216(5113):346–349. doi: 10.1038/216346a0. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Boman H. G. Resistance of Escherichia coli to penicillins. V. Physiological comparison of two isogenic strains, one with chromosomally and one with episomally mediated ampicillin resistance. J Bacteriol. 1968 Aug;96(2):438–446. doi: 10.1128/jb.96.2.438-446.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Ultraviolet radiation studies of filamentous Escherichia coli B. J Bacteriol. 1966 Oct;92(4):1062–1069. doi: 10.1128/jb.92.4.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Lieberman M. M., Rosenbaum N. Derepression of phosphomannose isomerase by regulator gene mutations involved in capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1967 Nov;94(5):1497–1501. doi: 10.1128/jb.94.5.1497-1501.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Carr H. S., Rose H. M. Electron microscopy of chloramphenicol-treated Escherichia coli. J Bacteriol. 1967 Jun;93(6):1987–2002. doi: 10.1128/jb.93.6.1987-2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. Genetic analysis of some mutations causing resistance to tetracycline in Escherichia coli K12. Genet Res. 1968 Jun;11(3):303–309. doi: 10.1017/s0016672300011484. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Suit J. C., Barbee T., Jetton S. Morphological changes in Escherichia coli strain C produced by treatments affecting deoxyribonucleic acid synthesis. J Gen Microbiol. 1967 Oct;49(1):165–173. doi: 10.1099/00221287-49-1-165. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]