Abstract
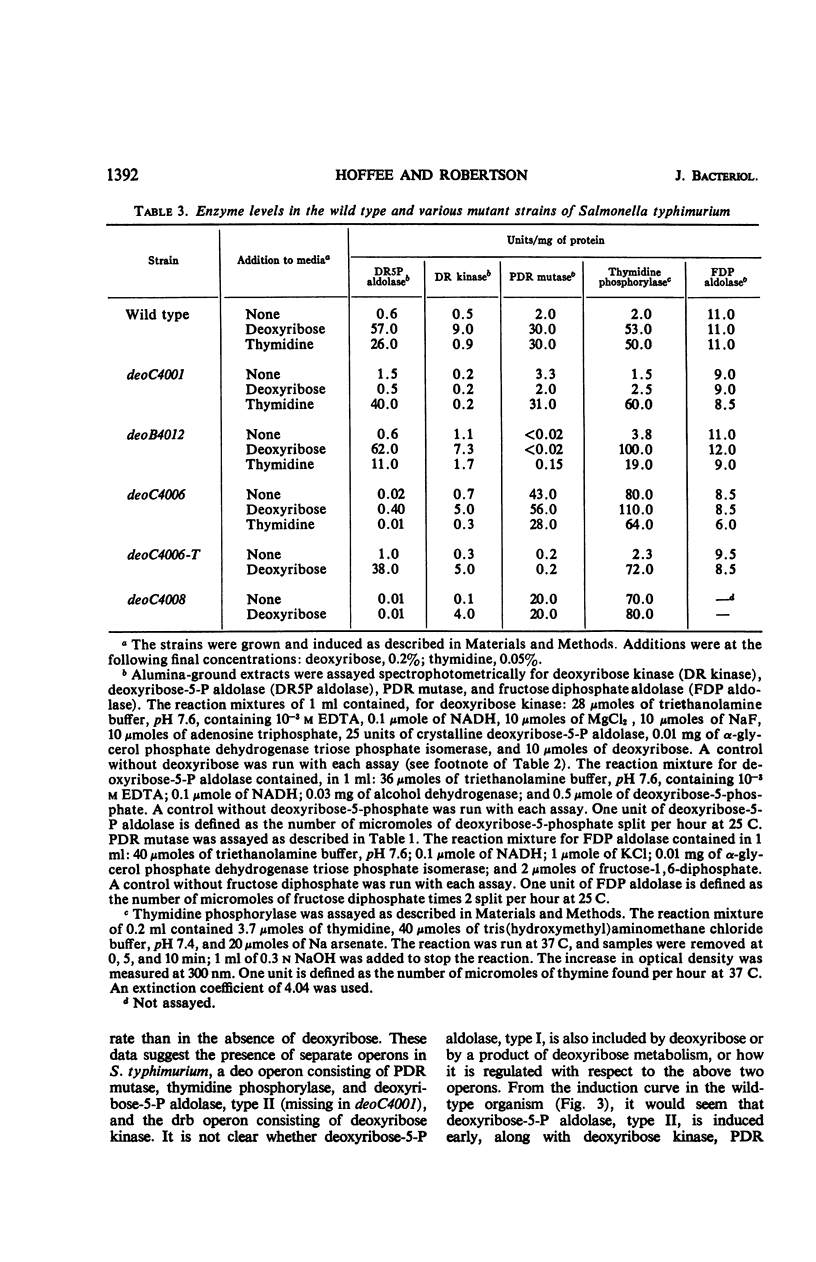
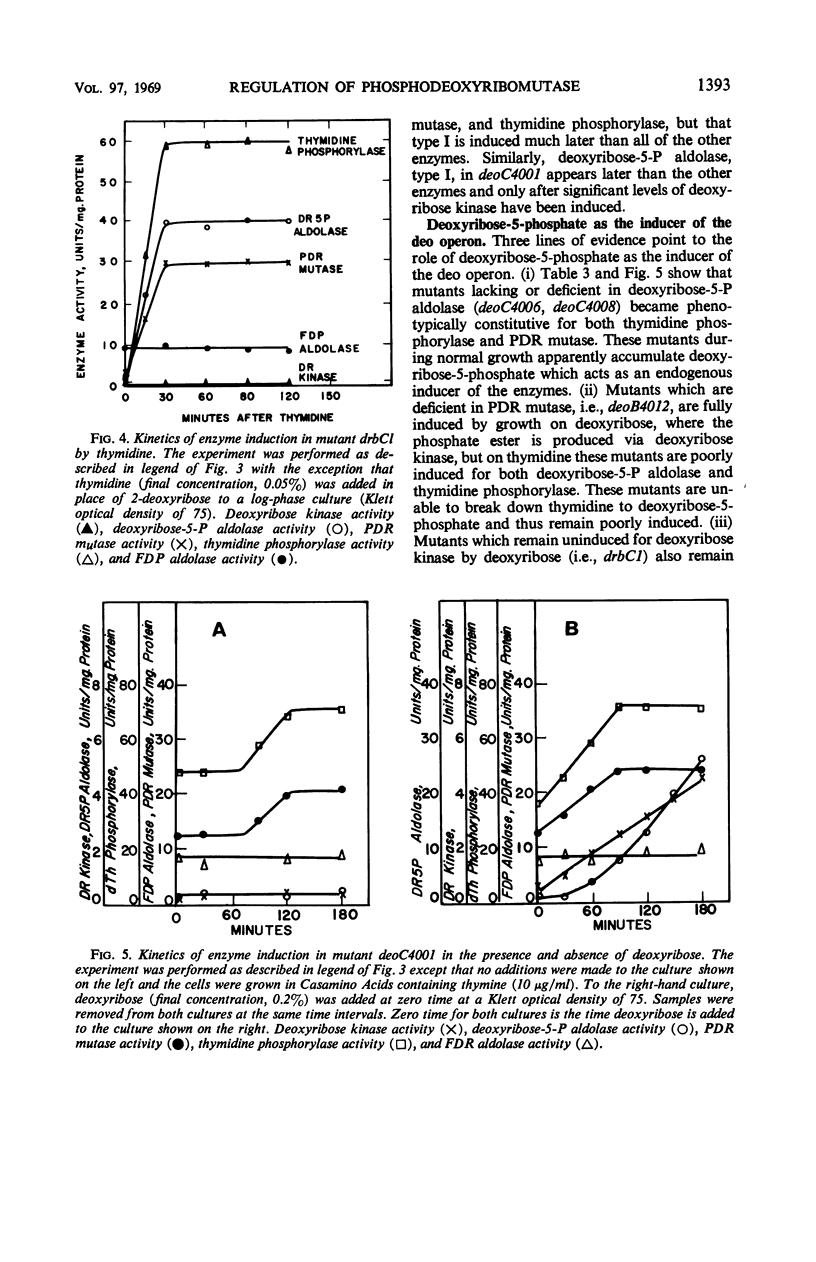
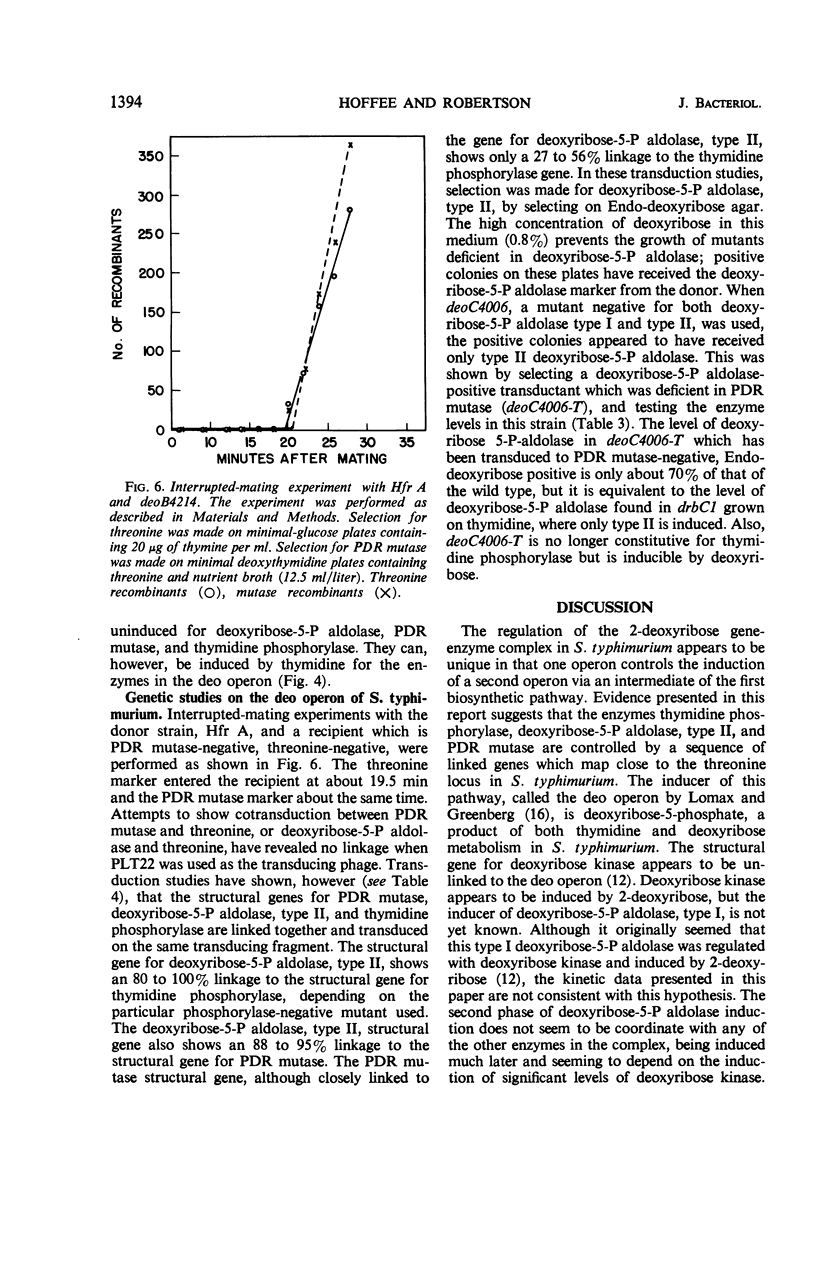
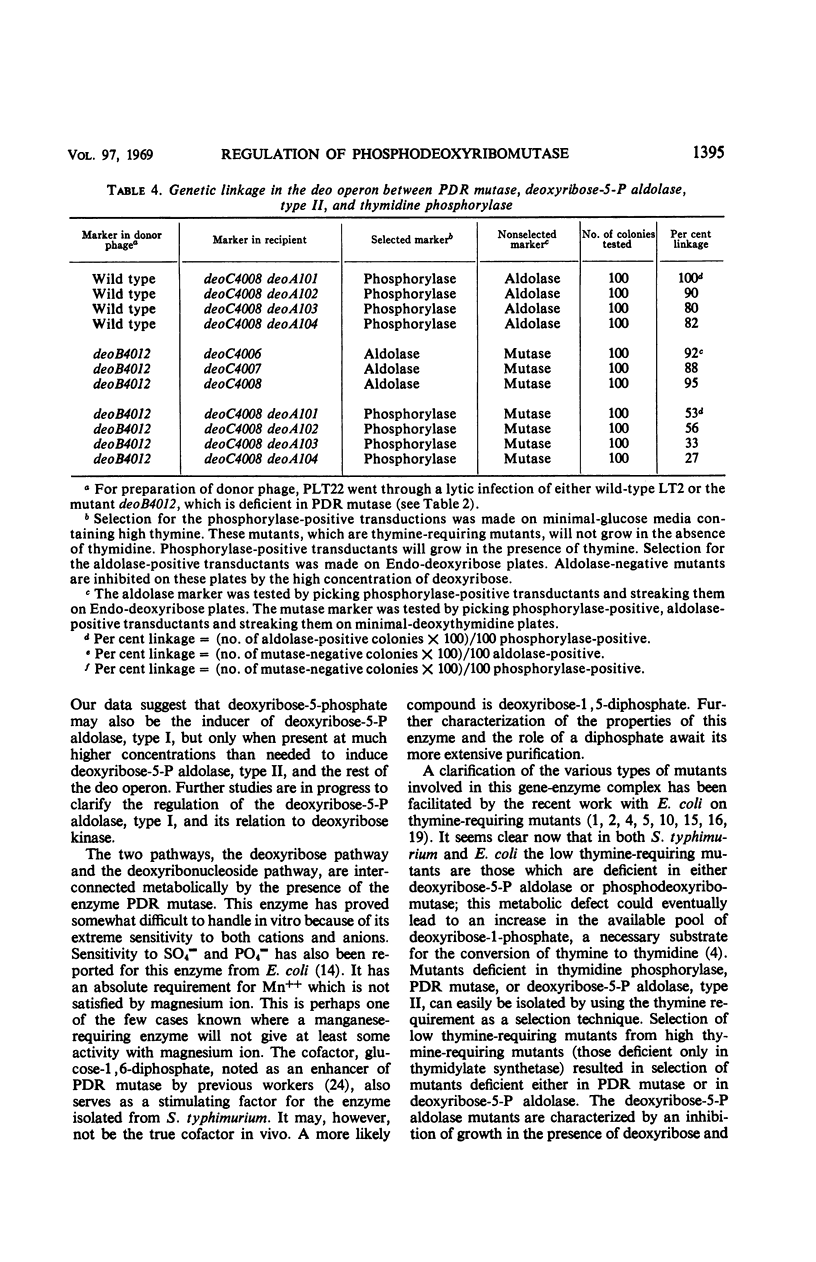
Phosphodeoxyribomutase, the enzyme which catalyzes the interconversion of 2-deoxyribose-1-phosphate to 2-deoxyribose-5-phosphate, has been partially purified from Salmonella typhimurium. The enzyme had an absolute requirement for manganese ion and was stimulated by glucose-1, 6-diphosphate. Phosphodeoxyribomutase was induced by deoxyribose-5-phosphate and was coordinately regulated with the enzymes thymidine phosphorylase and deoxyribose-5-phosphate aldolase, type II. Mutants deficient in these three enzymes were isolated and mapped close to the threonine locus in S. typhimurium. The three enzymes thymidine phosphorylase, deoxyribose-5-phosphate aldolase, type II, and phosphodeoxyribomutase are controlled by a series of linked genes and appear to constitute an operon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alikhanian S. I., Iljina T. S., Kaliaeva E. S., Kameneva S. V., Sukhodolec V. V. A genetical study of thymineless mutants of E. coli K12. Genet Res. 1966 Aug;8(1):83–100. doi: 10.1017/s0016672300009939. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Beacham I. R., Ahmad S. I., Pritchard R. H. The inducer of the deoxynucleoside phosphorylases and deoxyriboaldolase in Escherichia coli. Biochim Biophys Acta. 1968 Jul 23;161(2):554–557. doi: 10.1016/0005-2787(68)90132-9. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Eisenstark A., Barth P. T., Pritchard R. H. Deoxynucleoside-sensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1968;102(2):112–127. doi: 10.1007/BF01789138. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Bradford R. M. Inability of low thymine-requiring mutants of Escherichia coli lacking phosphodeoxyribomutase to be induced for deoxythymidine phosphorylase and deoxyriboaldolase. J Bacteriol. 1968 Jun;95(6):2434–2435. doi: 10.1128/jb.95.6.2434-2435.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Bradford R. M. The absence of deoxyriboaldolase activity in a thymineless mutant of Escherichia coli strain 15: a possible explanation for the low thymine requirement of some thymineless strains. Biochim Biophys Acta. 1967 Mar 29;138(1):217–220. doi: 10.1016/0005-2787(67)90610-7. [DOI] [PubMed] [Google Scholar]
- Dale B., Greenberg G. R. Genetic mapping of a mutation in Escherichia coli showing reduced activity of thymidine phosphorylase. J Bacteriol. 1967 Sep;94(3):778–779. doi: 10.1128/jb.94.3.778-779.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A., Eisenstark R., Cunningham S. Genetic analysis of thymineless(thy) mutants in Salmonella typhimurium. Genetics. 1968 Apr;58(4):493–506. doi: 10.1093/genetics/58.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., LOPER J. C., SERMAN D. Fine structure mapping by complete transduction between histidine-requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:323–353. doi: 10.1099/00221287-22-2-323. [DOI] [PubMed] [Google Scholar]
- HOFFMANN C. E., LAMPEN J. O. Products of desoxyribose degradation by Escherichia coli. J Biol Chem. 1952 Oct;198(2):885–893. [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- Hoffee P. A. 2-deoxyribose gene-enzyme complex in Salmonella typhimurium. I. Isolation and enzymatic characterization of 2-deoxyribose-negative mutants. J Bacteriol. 1968 Feb;95(2):449–457. doi: 10.1128/jb.95.2.449-457.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffee P. A. 2-deoxyribose-5-phosphate aldolase of Salmonella typhimurium: purification and properties. Arch Biochem Biophys. 1968 Sep 10;126(3):795–802. doi: 10.1016/0003-9861(68)90473-6. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lomax M. S., Greenberg G. R. Characteristics of the deo operon: role in thymine utilization and sensitivity to deoxyribonucleosides. J Bacteriol. 1968 Aug;96(2):501–514. doi: 10.1128/jb.96.2.501-514.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A. Thymineless mutants of Escherichia coli with deficiencies in deoxyribomutase and deoxyriboaldolase. Biochim Biophys Acta. 1968 Jun 18;161(1):279–282. doi: 10.1016/0005-2787(68)90325-0. [DOI] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem. 1952 May;196(1):347–365. [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- SMITH C. G., BERNSTEIN I. A. Studies on phosphodeoxyribomutase. Biochim Biophys Acta. 1961 Sep 2;52:184–193. doi: 10.1016/0006-3002(61)90916-7. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


