Abstract
Vitamin E (α-tocopherol) is a fat-soluble antioxidant that is transported by plasma lipoproteins in the body. α-Tocopherol taken up by the liver with lipoprotein is thought to be resecreted into the plasma in very low density lipoprotein (VLDL). α-Tocopherol transfer protein (αTTP), which was recently identified as a product of the causative gene for familial isolated vitamin E deficiency, is a cytosolic liver protein and plays an important role in the efficient recycling of plasma vitamin E. To throw light on the mechanism of αTTP-mediated α-tocopherol transfer in the liver cell, we devised an assay system using the hepatoma cell line McARH7777. Using this system, we found that the secretion of α-tocopherol was more efficient in cells expressing αTTP than in matched cells lacking αTTP. Brefeldin A, which effectively inhibits VLDL secretion by disrupting the Golgi apparatus, had no effect on α-tocopherol secretion, indicating that αTTP-mediated α-tocopherol secretion is not coupled to VLDL secretion. Among other agents tested, only 25-hydroxycholesterol, a modulator of cholesterol metabolism, inhibited α-tocopherol secretion. This inhibition is most likely mediated by oxysterol-binding protein. These results suggest that αTTP present in the liver cytosol functions to stimulate secretion of cellular α-tocopherol into the extracellular medium and that the reaction utilizes a novel non-Golgi-mediated pathway that may be linked to cellular cholesterol metabolism and/or transport.
α-Tocopherol is the most biologically active form of vitamin E and is an important antioxidant in cell membranes (1). It is an essential nutrient, and the major symptoms of vitamin E deficiency are neurological dysfunction, muscular weakness, and reproductive failures (2). α-Tocopherol, because of its hydrophobicity, requires special transport mechanisms in the aqueous environment of the plasma, body fluids, and cells. Unlike other fat-soluble vitamins, α-tocopherol has no specific plasma transport protein, but rather is transported by plasma lipoproteins (3). α-Tocopherol taken up by the intestine is incorporated into chylomicrons and delivered to the liver as chylomicron remnants. α-Tocopherol present in circulating lipoproteins such as low density lipoprotein (LDL) is also endocytosed to a great extent by the liver. Endocytosed lipoproteins are transported to the lysosomes, where the lipoprotein is hydrolyzed and free α-tocopherol is released. This α-tocopherol is repackaged in the liver cells and secreted into the plasma in nascent very low density lipoprotein (VLDL). These processes ensure the efficient recycling of plasma α-tocopherol (4).
α-Tocopherol transfer protein (αTTP) is a cytosolic liver protein with a highly specific binding affinity for α-tocopherol. αTTP has also been shown to have the ability to transport α-tocopherol between membranes by an in vitro transfer assay using liposomes and liver membrane fraction (5, 6). We have purified this protein from rat liver and isolated its cDNA from rats and humans (7–9). Recently, we have demonstrated that mutations in the gene that encodes αTTP cause ataxia with isolated vitamin E deficiency (AVED) (10, 11). Patients with AVED have low or undetectable serum α-tocopherol concentrations and progressive spinocerebellar dysfunction. In these patients, the absorption of α-tocopherol from the intestine and its delivery to the liver are normal, but they have an impaired ability to incorporate α-tocopherol into VLDL in the liver (3). From these observations, it was suggested that αTTP catalyzes the transfer of α-tocopherol taken up by the liver into nascent VLDL; however, little is known about the mechanisms underlying αTTP-mediated intracellular transport of α-tocopherol in liver cells.
In the present study, we made stable transfectants of the rat hepatoma cell line McARH7777 that express rat αTTP, and we devised an assay to examine the role of αTTP in the transport of α-tocopherol in the cells. Using this system, we provide the evidence that αTTP stimulates α-tocopherol secretion from McARH7777 cells and that the secretion of α-tocopherol is through a non-Golgi-mediated pathway, which is modulated by oxysterols.
METHODS
Materials.
[14C]Paraformaldehyde (50 mCi/mmol; 1 mCi = 37 MBq) and [14C]oleate (50 mCi/mmol) were purchased from Amersham. γ-Tocopherol was a gift from Eisai (Tokyo). Lipids were purchased from Avanti Polar Lipids. The mammalian expression vector pcDNA3 was purchased from Invitrogen. Brefeldin A (BFA) was purchased from Wako Pure Chemical (Osaka). 25-Hydroxycholesterol, 20-hydroxycholesterol, and 7-ketocholesterol were purchased from Sigma.
Cell Culture.
McARH7777 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 10% horse serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine, in 5% CO2/95% air at 37°C. The cell cultures were split twice a week and fed every day.
Synthesis of α-[14C]Tocopheryl Acetate.
α-[14C]Tocopheryl acetate was synthesized as previously described (12) with slight modifications. Hydroxy[14C]methylation of d-γ-tocopherol (72 mg, 0.17 mmol) was performed with [14C]paraformaldehyde (12.5 mCi, 50 mCi/mmol), boric acid (13 mg, 0.2 mmol), and one drop of acetic acid in toluene (0.6 ml), and the mixture was stirred at 110°C for 3 h under nitrogen. The reaction mixture was washed with water four times. Reduction of 5-hydroxy[14C]methyl-α-tocopherol was performed by adding acetic acid (0.8 ml), hydrochloric acid (1.2 ml), and zinc dust (0.13 g) to the reaction mixture (10 ml), and stirring the mixture vigorously for 3 h at room temperature. The toluene layer was separated, washed twice with water, twice with 5% aqueous NaHCO3, and twice with water.
Acetylation of 5-[14C]methyl-α-tocopherol (0.77 mg, 100 μCi) was performed with acetic anhydride (6.0 mg) and N,N-dimethylaminopyridine (3.5 mg) in anhydrous CHCl3 (0.9 ml) at 50°C overnight. The reaction mixture was extracted by the method of Bligh and Dyer (13), and α-[14C]tocopheryl acetate was purified on thin-layer chromatography with a developing system of hexane/diethyl ether/acetic acid (55:45:1, vol/vol).
Preparation of Liposomes.
Multilamellar liposomes were prepared as described previously (14). The liposomes were composed of phosphatidylcholine/phosphatidylserine/dicetyl phosphate/cholesterol/α-[14C]tocopheryl acetate (50 mCi/mmol) (molar ratio 50:50:10:75:2). The mixture of these lipids was dried in a rotary evaporator under reduced pressure, and the dried lipids were resuspended in 0.3 M glucose at room temperature.
Preparation of [14C]Oleate–BSA Complex.
[14C]Oleate–BSA complex was prepared as described previously (15). An aliquot of [14C]oleate (10 μCi) was evaporated to dryness under a stream of nitrogen. The [14C]oleate was resuspended in 0.8 ml of 3% (wt/vol) BSA in DMEM, and the solution was stirred at room temperature.
Establishment of αTTP Stable Transformants.
An expression vector containing full-length rat αTTP and the neomycin-resistance gene, pcDNA3-αTTP, was constructed and transfected into McARH7777 cells. Colonies resistant to G418 (500 μg/ml) were isolated and screened for the expression of αTTP by immunoblotting. One of these transformants, named McA-TTP21, was used in the following experiments.
Incubation of Cells with Radiolabeled Lipids.
Cells were plated at a density of 5 × 104 cells per cm2 into 24-well collagen-coated plates. Cell monolayers were grown until approximately confluent. The medium was removed, and cells were incubated for the indicated time at 37°C in 0.5 ml of medium with α-[14C]tocopheryl acetate-containing liposomes (0.05 μCi per well). To label the triglyceride, cells were incubated in the presence of [14C]oleate–BSA complex (0.5 μCi per well).
To determine the mechanism of αTTP-mediated α-tocopherol secretion, cells were incubated with radiolabeled lipids in the presence of the indicated agents (dissolved in ethanol) for the indicated times.
α-tocopherol secreted (%) was calculated as follows: α-tocopherol secretion (%) = [α-[14C]tocopherol in medium ÷ (α-[14C]tocopherol in cell + α-[14C]tocopherol in medium)] × 100 (%).
In a typical experiment, about 120 pmol of α-[14C]tocopherol per 1 × 105 cells per 24 h was produced from α-[14C]tocopheryl acetate in McA-TTP21 cells, and about 80% of it (100 pmol) was secreted into the medium during 24-h incubation.
Lipid Extraction and Analysis by Thin-Layer Chromatography.
After incubation, extracellular and cellular lipids were extracted by hexane. Cells were washed twice with phosphate-buffered saline (PBS) and were lysed by adding 0.1 ml of 0.1% sodium dodecyl sulfate (SDS). An aliquot was diluted with 0.4 ml of water and used for the following procedure. The cell lysate or medium (0.5 ml) was added to 1 ml of 6% pyrogallol in ethanol. The mixture was diluted with 1.5 ml of 65% ethanol. Three milliliters of hexane was added and the mixture was mixed vigorously in a Vortex mixer before being centrifuged at room temperature. The hexane layer was saved, and the hexane extracts were evaporated under nitrogen. The residue was redissolved in ethyl acetate and subjected to thin-layer chromatography on a silica gel plate. The plate was developed with hexane/diethyl ether/acetic acid (55/45/1). After development, plates were exposed and analyzed, using a bio-image analyzer (Fuji Film).
RESULTS
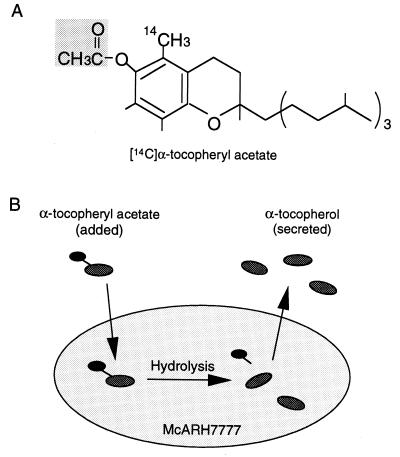
It has been hypothesized in previous reports that αTTP catalyzes the intracellular transport of α-tocopherol taken up by the liver cells into nascent VLDL for secretion (3). We sought to reproduce these processes in a cell culture system using radiolabeled α-tocopheryl acetate (Fig. 1A), which is thought to be hydrolyzed to generate α-tocopherol when entering cells, to examine the secretion of α-tocopherol from cultured cells. This α-tocopherol will be secreted into the medium by the action of αTTP. Thus it should be possible to evaluate the activity of αTTP in cultured cells by measuring the amount of α-tocopherol appearing in the extracellular medium (Fig. 1B).
Figure 1.
(A) Structure of α-[14C]tocopheryl acetate. (B) Assay of α-tocopherol secretion from McARH7777 cells.
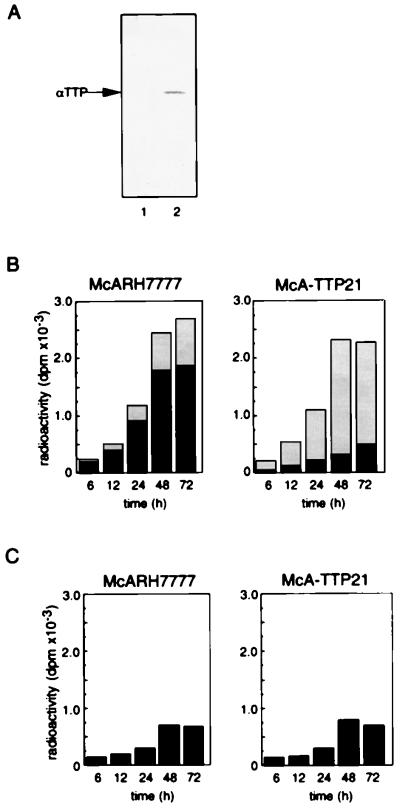
Previous studies have demonstrated that αTTP expression decreases dramatically within 24 h when rat liver parenchymal cells are transferred to in vitro primary culture (16). In addition, we have observed that αTTP expression levels in some liver tumor cell lines such as HepG2 and McARH7777, both of which have the biosynthetic capabilities of normal liver parenchymal cells (17–19), are extremely low (unpublished observations). In this study, we chose the rat hepatoma cell line McARH7777, and we prepared McARH7777 variant cells that constitutively express rat αTTP by transfecting its cDNA. Western blot analysis of αTTP in McA-TTP21, one of the transformed cell lines, demonstrated the expression of αTTP at a level comparable to that in freshly isolated rat hepatocytes (Fig. 2A).
Figure 2.
Effect of expression of αTTP on the secretion of α-tocopherol from McARH7777 cells. (A) Expression of αTTP by transformed McARH7777 cells. McARH7777 cells were stably transfected with an αTTP expression plasmid. After selection in medium supplemented with G418, surviving colonies were subcultured and the level of αTTP was assessed by Western blotting using an anti-αTTP polyclonal antibody. The transfected cells were named McA-TTP, and one of these transformants, named McA-TTP21, was tested. Lane 1, McARH7777; lane 2, McA-TTP21. (B) McARH7777 and McA-TTP21 cell monolayers were incubated with α-[14C]tocopheryl acetate in liposomes. After incubation for the indicated times, the amount of α-[14C]tocopherol in the cell (black) and medium (shaded) fractions were measured. (C) The amounts of α-[14C]tocopheryl acetate in the cell fraction were also measured.
There was no ester hydrolysis in the absence of cells, but in the presence of cells, α-[14C]tocopheryl acetate was hydrolyzed to α-[14C]tocopherol in a time-dependent manner (data not shown). When incubated with α-[14C]tocopheryl acetate-containing liposomes, McARH7777 and McA-TTP21 cells showed similar rates of α-[14C]tocopheryl acetate hydrolysis. Fig. 2B shows the amounts of α-[14C]tocopherol in the cellular and extracellular fractions during the incubation. About 20% of the α-[14C]tocopherol accumulated in the medium of control McARH7777 cells, and the rest was with the cells. In marked contrast, more than 70% of the α-[14C]tocopherol was recovered from the medium of McA-TTP21 cells. On the other hand, the levels of α-[14C]tocopheryl acetate, which is a poor substrate for αTTP (7), associated with the cells did not show a significant difference between McARH7777 cells and McA-TTP21 cells (Fig. 2C). These results clearly demonstrate that αTTP stimulates α-tocopherol secretion from McARH7777 cells into the medium.
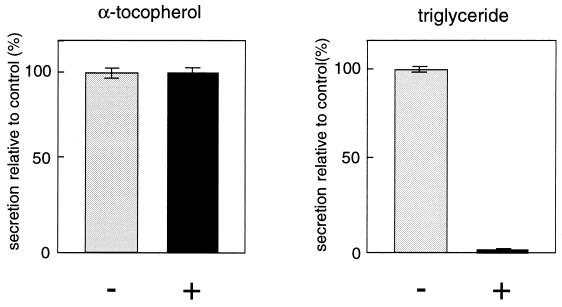
It has previously been postulated that α-tocopherol is secreted from the liver in association with VLDL by in vivo and liver perfusion experiments (3). To explore the mechanism of αTTP-mediated α-tocopherol secretion, we tested the effect of BFA, a known inhibitor of VLDL secretion that disrupts the Golgi apparatus (19). McA-TTP21 cells were incubated with both α-[14C]tocopheryl acetate and [14C]oleate in the presence or absence of 1.0 μg/ml BFA, and the amounts of α-[14C]tocopherol and [14C]triglyceride secreted were determined after 24 h. Triglyceride is a major lipid component of VLDL. Although triglyceride secretion was significantly inhibited, the secretion of α-tocopherol was not affected to any extent by this reagent (Fig. 3). Monensin, another inhibitor of lipoprotein secretion (20), had no significant effect on αTTP-mediated α-tocopherol secretion in our assay system (data not shown). These data suggested that the secretion of α-tocopherol is independent of VLDL secretion, and it uses a non-Golgi-mediated pathway. It was also found, by NaBr density gradient analysis, that α-tocopherol secreted into the medium in the presence of BFA was associated with lipoproteins present in the culture medium (data not shown).
Figure 3.
Effect of BFA on the secretion of α-tocopherol from McA-TTP21 cells. McA-TTP21 cell monolayers were incubated with α-[14C]tocopheryl acetate and [14C]oleate in the presence (+) or absence (−) of BFA (1.0 μg/ml). After a 24-h incubation, the radioactivities corresponding to α-tocopherol and triglyceride of the cell and medium fractions were measured. The results are expressed as percentages of the secretion level obtained in the absence of BFA. Each point represents the mean ± SE of triplicate determinations.
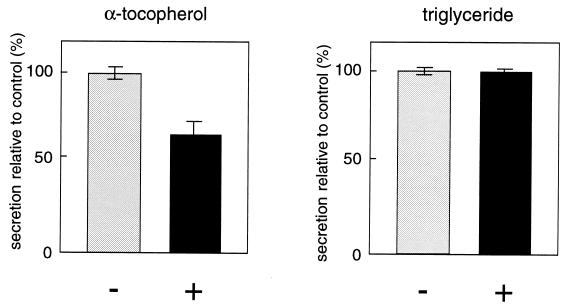
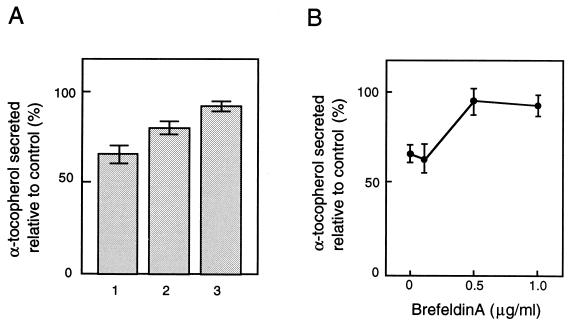
We then tried to find out which drugs affect αTTP-stimulated α-tocopherol secretion. Among the agents tested, 2.5 μg/ml 25-hydroxycholesterol partially but significantly inhibited the secretion of α-tocopherol, whereas triglyceride secretion was not affected (Fig. 4), again indicating the uncoupling of VLDL and α-tocopherol secretion. Oxysterols, such as 25-hydroxycholesterol, are known to elicit a series of well defined regulatory responses in cultured cells that culminate in the inhibition of de novo cholesterol synthesis (21). A high-affinity binding protein for oxysterols, oxysterol-binding protein (OSBP), has been identified which displays affinities for various oxysterols that are proportional to their ability to suppress cholesterol synthesis in cultured cells (22, 23). To determine if OSBP mediates the action of 25-hydroxycholesterol on α-tocopherol secretion, we compared the inhibitory effects of three OSBP ligands with different binding affinities: 25-hydroxycholesterol, Kd = 0.03 μM; 20-hydroxycholesterol, Kd = 0.06 μM; and 7-ketocholesterol, Kd = 1.4 μM (Fig. 5A). A correlation was suggested between OSBP binding affinities and suppression of α-tocopherol secretion for these oxysterols. Recently it was reported that OSBP undergoes translocation from a cytoplasmic/vesicular compartment to the Golgi apparatus in the presence of ligand, and that an intact Golgi apparatus is required for OSBP-mediated suppression of mRNAs for several sterol-regulated genes, based on the observation that BFA treatment abolished the effect of 25-hydroxycholesterol (24, 25). We also tested whether BFA disrupts the inhibition by 25-hydroxycholesterol of α-tocopherol secretion (Fig. 5B). BFA, when added to the cells 15 min prior to the addition of 25-hydroxycholesterol, prevented 25-hydroxycholesterol-mediated suppression of α-tocopherol secretion in a dose-dependent fashion. The relative level of α-tocopherol secretion returned to its highest value in the presence of 0.5 μg/ml BFA.
Figure 4.
Effect of 25-hydroxycholesterol on the secretion of α-tocopherol from McA-TTP21 cells. McA-TTP21 cell monolayers were incubated with α-[14C]tocopheryl acetate and [14C]oleate in the presence (+) or absence (−) of 25-hydroxycholesterol (2.5 μg/ml). After a 24-h incubation, the radioactivities corresponding to α-tocopherol and triglyceride of the cell and medium fractions were measured. The results are expressed as percentages of the secretion level obtained in the absence of 25-hydroxycholesterol. Each point represents the mean ± SE of triplicate determinations.
Figure 5.
Effect of 25-hydroxycholesterol is probably mediated by OSBP. (A) Effects of various oxysterols on the secretion of α-tocopherol from McA-TTP21 cells. McA-TTP21 cells were incubated in the presence of 25-hydroxycholesterol (2.5 μg/ml, lane 1), 20-hydroxycholesterol (lane 2), or 7-ketocholesterol (lane 3). After a 24-h incubation, the radioactivities of the cell and medium fractions were measured. The results are expressed relative to values for cells incubated without additives. Each point represents the mean ± SE of triplicate determinations. (B) 25-Hydroxycholesterol-mediated suppression of the secretion of α-tocopherol from McA-TTP21 cells in the presence of BFA. McA-TTP21 cells were incubated in the presence of 25-hydroxycholesterol (2.5 μg/ml) with various concentrations of BFA. After a 24-h incubation, the radioactivities of the cell and medium fractions were measured. The results are expressed relative to values for cells incubated without additives. Each point represents the mean ± SE of triplicate determinations.
DISCUSSION
In the present study, we established an assay system using the hepatoma cell line McARH7777 for the secretion of α-tocopherol from liver cells. Using this system, we demonstrated that αTTP stimulated the secretion of α-tocopherol from the liver cells. Furthermore, we also found that αTTP-mediated α-tocopherol secretion is not coupled to VLDL secretion and instead utilizes a novel non-Golgi-mediated pathway that is sensitive to 25-hydroxycholesterol. Previous reports of in vivo and liver perfusion experiments have suggested that α-tocopherol secretion is coupled to VLDL secretion, since α-tocopherol secreted from the liver is associated with nascent VLDL (20, 26, 27). This idea is supported by the fact that patients with abetalipoproteinaemia, who have virtually undetectable plasma levels of lipoproteins containing apolipoprotein B (chylomicrons, VLDL, and LDL), exhibit severe vitamin E deficiency (3). BFA has been shown to block the intracellular transfer and secretion of proteins. Olofsson and co-workers (19) have shown that secretion of apoB48 and apoB100, both of which are protein components of rat VLDL, and transferrin from cultured McARH7777 cells was completely inhibited by 1.0 μg/ml BFA. Consistent with this result, we observed that treatment of McARH7777 cells with 1.0 μg/ml BFA caused complete inhibition of triglyceride secretion (Fig. 3), however, αTTP-mediated α-tocopherol secretion from the liver cells was not affected to any extent by this treatment. Our results suggest that α-tocopherol is not assembled into VLDL within the cell. Although the data obtained from different experimental systems (27) appear to be inconsistent, it is possible that α-tocopherol secreted by liver cells becomes associated with nascent VLDL in the sinusoidal spaces of the liver before entering the circulation. Recently, plasma phospholipid transfer protein (PLTP) was reported to accelerate the exchange/transfer of α-tocopherol between different lipoproteins and between lipoproteins and cells (28). Extracellular plasma proteins such as PLTP might facilitate this process.
The nature of the pathway involved in α-tocopherol secretion was suggested by the observation that partial but significant suppression of α-tocopherol secretion was caused by 25-hydroxycholesterol treatment. The observed effect of 25-hydroxycholesterol may not result from direct inhibiton of αTTP by this reagent for the following reasons: (i) 25-Hydroxycholesterol has no effect on αTTP-mediated α-tocopherol transfer between two membranes in an in vitro system (data not shown). (ii) BFA, which did not show any appreciable effect on αTTP-mediated α-tocopherol secretion, completely reversed the action of 25-hydroxycholesterol. 25-Hydroxycholesterol regulates sterol metabolism by transcriptional and post-transcriptional effects. OSBP, a cytosolic 90-kDa protein, is the candidate that mediates the regulatory actions of 25-hydroxycholesterol. Ridgway and Lagace (25) showed that upon addition of 25-hydroxycholesterol, most of the OSBP becomes concentrated in the Golgi apparatus and that BFA completely suppresses the action of 25-hydroxycholesterol. Our results strongly support the concept that inhibition by 25-hydroxycholesterol of α-tocopherol secretion is through OSBP. Although 25-hydroxycholesterol may have effect(s) other than on sterol metabolism, it is reasonable to assume that α-tocopherol secretion is connected to cellular cholesterol metabolism and/or transport. A finding that may support this hypothesis is the observation that BFA does not inhibit the transport of newly synthesized cholesterol from the endoplasmic reticulum to the plasma membrane (29, 30). Transport of newly synthesized cholesterol from the endoplasmic reticulum to the plasma membrane is an energy-dependent process, and energy depletion results in accumulation of newly synthesized cholesterol in the endoplasmic reticulum. Cholesterol transport is also interrupted by incubation of cells at 15°C, resulting in accumulation of newly synthesized cholesterol in the endoplasmic reticulum and in a subcellular fraction of vesicles of low density. It is proposed that this lipid-rich vesicle fraction contains an intermediate in the intracellular transport of cholesterol from the site of synthesis in the endoplasmic reticulum to the final destination in the plasma membrane. BFA does not affect cholesterol transport to the plasma membrane; thus it is postulated that cholesterol-containing vesicles bypass the Golgi apparatus and proceed to the plasma membrane. Such vesicles might be involved in the transport of α-tocopherol out of the cells. We recently identified a membrane-associated 50-kDa protein that specifically interacts with a glutathione S-transferase (GST)-αTTP recombinant fusion protein but not with GST (preliminary observations). Identification of such protein(s) may help to further characterize the machinery involved in the intracellular transport of α-tocopherol.
Acknowledgments
M.A. is a recipient of a Fellowship of the Japan Society for the Promotion of Science for Japanese Junior Scientists.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: LDL, low density lipoprotein; VLDL, very low density lipoprotein; αTTP, α-tocopherol transfer protein; BFA, brefeldin A; OSBP, oxysterol-binding protein.
References
- 1.Burton G W, Ingold K U. Acc Chem Res. 1986;19:194–201. [Google Scholar]
- 2.Sokol R J. Annu Rev Nutr. 1988;8:351–373. doi: 10.1146/annurev.nu.08.070188.002031. [DOI] [PubMed] [Google Scholar]
- 3.Kayden H J, Traber M G. J Lipid Res. 1993;34:343–358. [PubMed] [Google Scholar]
- 4.Traber M G, Ramakrishnan R, Kayden H J. Proc Natl Acad Sci USA. 1994;91:10005–10008. doi: 10.1073/pnas.91.21.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowri H, Nakagawa Y, Inoue K, Nojima S. Eur J Biochem. 1981;117:537–542. doi: 10.1111/j.1432-1033.1981.tb06370.x. [DOI] [PubMed] [Google Scholar]
- 6.Bloj B, Zilversmit D B. J Biol Chem. 1977;252:1613–1619. [PubMed] [Google Scholar]
- 7.Sato Y, Hagiwara K, Arai H, Inoue K. FEBS Lett. 1991;288:41–45. doi: 10.1016/0014-5793(91)80999-j. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Arai H, Miyata A, Tokita S, Yamamoto K, Tanabe T, Inoue K. J Biol Chem. 1993;268:17705–17710. [PubMed] [Google Scholar]
- 9.Arita M, Sato Y, Miyata A, Tanabe T, Takahashi E, Kayden H J, Arai H, Inoue K. Biochem J. 1995;306:437–443. doi: 10.1042/bj3060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouahchi K, Arita M, Kayden H J, Hentati F, Hamida M B, Sokol R, Arai H, Inoue K, Mandel J L, Koenig M. Nat Genet. 1995;9:141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 11.Gotoda T, Arita M, Arai H, Inoue K, Yokota T, Fukuo Y, Yazaki Y, Yamada N. New Engl J Med. 1995;333:1313–1318. doi: 10.1056/NEJM199511163332003. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Kijima S. Chem Pharm Bull. 1972;20:1681–1686. [Google Scholar]
- 13.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 14.Furuchi T, Aikawa K, Arai H, Inoue K. J Biol Chem. 1993;268:27345–27348. [PubMed] [Google Scholar]
- 15.Goldstein J L, Basu S K, Brown M S. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim H S, Arai H, Arita M, Sato Y, Ogihara T, Tamai H, Inoue K, Mino M. J Nutr Sci Vitaminol. 1996;42:11–18. doi: 10.3177/jnsv.42.11. [DOI] [PubMed] [Google Scholar]
- 17.White A L, Graham L G, LeGros J, Pease R J, Scott J. J Biol Chem. 1992;267:15657–15664. [PubMed] [Google Scholar]
- 18.Boren J, Rustaeus S, Olofsson S O. J Biol Chem. 1994;269:25879–25888. [PubMed] [Google Scholar]
- 19.Rustaeus S, Lindberg K, Boren J, Olofsson S O. J Biol Chem. 1995;270:28879–28886. doi: 10.1074/jbc.270.48.28879. [DOI] [PubMed] [Google Scholar]
- 20.Bjorneboe A, Bjorneboe G A, Hagen B F, Nossen J O, Drevon C A. Biochim Biophys Acta. 1987;922:199–205. doi: 10.1016/0005-2760(87)90155-x. [DOI] [PubMed] [Google Scholar]
- 21.Kandutsch A A, Chen H W, Heiniger H J. Science. 1978;201:498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- 22.Taylor F, Saucier S, Shown E, Parish E, Kandutsch A A. J Biol Chem. 1984;259:12382–12387. [PubMed] [Google Scholar]
- 23.Dawson P A, Ridgway N D, Slaughter C A, Brown M S, Goldstein J L. J Biol Chem. 1989;264:16798–16803. [PubMed] [Google Scholar]
- 24.Ridgway N D, Dawson P A, Brown M S, Goldstein J L. J Cell Biol. 1992;116:307–319. doi: 10.1083/jcb.116.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridgway N D, Lagace T A. J Biol Chem. 1995;270:8023–8031. doi: 10.1074/jbc.270.14.8023. [DOI] [PubMed] [Google Scholar]
- 26.Traber M G, Rudel L L, Burton G W, Hughes L, Ingold U, Kayden H J. J Lipid Res. 1990;31:687–694. [PubMed] [Google Scholar]
- 27.Traber M G, Sokol R J, Burton G W, Ingold K U, Papas A M, Huffaker J E, Kayden H J. J Clin Invest. 1990;85:397–407. doi: 10.1172/JCI114452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostner G M, Oettl K, Jauhiainen M, Ehnholm C, Esterbauer H, Dieplinger H. Biochem J. 1995;305:659–667. doi: 10.1042/bj3050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urbani L, Simoni R D. J Biol Chem. 1990;265:1919–1923. [PubMed] [Google Scholar]
- 30.Liscum L, Underwood K W. J Biol Chem. 1995;270:15443–15446. doi: 10.1074/jbc.270.26.15443. [DOI] [PubMed] [Google Scholar]