Abstract
If behavioral isolation between species can evolve as a consequence of sexual selection within a species, then traits that are both sexually selected and used as a criterion of species recognition by females should be identifiable. The broad male head of the Hawaiian picture-winged fly Drosophila heteroneura is a novel sexual dimorphism that may be sexually selected and involved in behavioral isolation from D. silvestris. We found that males with broad heads are more successful in sexual selection, both through female mate choice and through aggressive interactions. However, female D. heteroneura do not discriminate against hybrids on the basis of their head width. Thus, this novel trait is sexually selected but is not a major contributor to species recognition. Our methods should be applicable to other species in which behavioral isolation is a factor.
The existence of behavioral reproductive isolation is well known, but its evolutionary origins are poorly understood (1). Behavioral isolation may form a continuum with sexual selection; behavioral isolation between species is thought to be mediated by discrimination against potential mates of the inappropriate species whereas sexual selection describes differential mating success within a species, often mediated by female mating preferences. This view of a continuum is supported by genetic models that show that behavioral isolation can evolve as a consequence of sexual selection within a species (2–4). On the other hand, Paterson (5) has proposed that sexual selection and species recognition are fundamentally different phenomena; he coined the term “specific-mate recognition system” to emphasize this difference. The specific-mate recognition system is thought to be invariant within a species. One way to assess these conflicting views of the role of sexual selection in speciation is to assess within-species variation for mating behavior in a variety of species (6). Another approach is to examine the processes of sexual selection and species recognition in great detail for a pair of species, as we describe below. Novel sexual dimorphisms are excellent candidates for being both sexually selected and involved in isolation. One such dimorphism, the broad male head of the Hawaiian picture-winged fly Drosophila heteroneura, has been suggested to be sexually selected and to play a role in behavioral isolation from D. silvestris (7, 8).
Two general classes of tests can be used to study the continuity of sexual selection and species recognition. One is to identify one or more traits that are sexually selected within one species and then to study the same traits as cues for species recognition. The other approach is the reverse, first to identify traits that are involved in species recognition and then ask whether they are sexually selected within either species. We illustrate the first approach with our study of head width in Hawaiian flies.
Evolution of the Hawaiian Drosophila is commonly treated as an example of sexual selection influencing speciation (7). D. heteroneura and its close relative D. silvestris are partially sympatric in cloud forests on the Island of Hawaii, where they occasionally hybridize (9, 10). Both species have non-resource-based mating systems that resemble exploded leks (8, 11): Males defend mating territories on the bare stipes of tree fern fronds, but adults and larvae feed on rotting tissue of woody plants in the families Lobeliaceae and Araliaceae. Thus, male aggression and male courtship are the major determinants of male mating success. The broad head of the male D. heteroneura distinguishes it from the male D. silvestris. Spieth (8) proposed that the broad head evolved concomitantly with a crouched fighting shove in D. heteroneura. Templeton (12) suggested that male head width was subject to strong stabilizing sexual selection through mate choice; females could detect the width of a male’s head because males stand closely in front of females during courtship. Although this proposition is repeated in several widely used textbooks (13–15), no analyses of the relationship between head width and courtship success or aggressive success have been published.
In the laboratory, reciprocal crosses between D. silvestris and D. heteroneura are fertile and show no postzygotic breakdown (16–18), but the cross between D. heteroneura females and D. silvestris males is hard to produce (16, 17, 19, 20). In the field, all hybrids are progeny from crosses of a D. silvestris female and a D. heteroneura male (10). The weak isolation between these species suggests that they are at an early stage of divergence, and thus novel traits may be contributing to divergence rather than having evolved after the gene pools became distinct. The courtship behavior of both species is very similar in terms of the acts performed and differs slightly in the timing and probabilities of different acts (21). However, these behavioral differences appear to have a minor influence on reproductive isolation because the failure to hybridize in the laboratory is because of the failure of courtship to begin after the male and female face each other not because of failure later in courtship (19). These observations suggest that female D. heteroneura may discriminate against heterospecific males because of the males’ small heads. Here, we describe tests for sexual selection on head width within D. heteroneura and a test of the hypothesis that male head width is a cue for species discrimination.
MATERIALS AND METHODS
We tested the hypothesis that male head width is subject to sexual selection in D. heteroneura by examining the two major contributors to sexual selection, courtship success and aggressive success, in separate experiments. Flies were from the stocks that are numbered Y11R6 and U26B9 (D. silvestris from the Upper Ola’a Forest Reserve, Puna District, and Kahuku Ranch, Kau District, Hawaii, respectively) and W48B6 (D. heteroneura from the western slope of Hualalai Volcano, North Kona District) at the Hawaii Drosophila Stock Center at the University of Hawaii. We report data from two stocks of D. silvestris because these represent two populations that differ in a male secondary sexual characteristic, the number of rows of bristles on the foretibiae (22); Y11R6 is from a three-row population, and U26B9 is from a two-row population. D. heteroneura males have two rows of bristles, which appears to be the ancestral state (22). Some studies have reported behavioral isolation between two-row and three-row populations of D. silvestris (18, 23), but the sign and magnitude of isolation between these populations appear to be sensitive to the type of behavioral test involved (20).
All behavioral tests were conducted during the peak activity period, between 1 and 4 h after the lights in the rearing room were turned on. We used no-choice tests because the territorial nature of the mating system dictates that females in the forest are unable to sample two males simultaneously. Each pair was watched for 1 h in a clear plastic observation chamber (15 × 12 × 4.5 cm) that had a floor covered with blotting paper and humidity provided by a dish containing a damp sponge; these chambers are large enough for unreceptive females to avoid courtship and mating (they remain motionless on the floor or perch near the top of the wall and are not encountered by the male).
For the tests of courtship success within D. heteroneura, we housed males in individual vials and tested them on each of 10 days with a different virgin female. The testing sequence lasted 11 or 12 days; as many as 12 chambers were watched simultaneously. We noted whether each male mated and scored his mating success as the number of copulations out of 10 tests. Thirty males were used.
For tests of aggression, we marked males individually by chilling them on crushed ice for 4 min, then painting a yellow dot on either the left or the right of the thorax. We observed two males per chamber for 1 h. These males were chosen without regard to size, subject to the constraint that one had a mark on his left and the other on his right. We noted the level of intensity of aggressive interactions and only kept data from the 29 pairs that had at least one high intensity encounter in which they crouched head-to-head with their wings extended laterally, tips nearly touching. Such fights usually had decisive outcomes, in which the winning male stood his ground and the other walked or ran away.
All of the males from the courtship tests and the males that were involved in high intensity fights were frozen until they were measured. They were pinned, and their body parts were measured to the nearest 0.05 mm with an ocular micrometer. We measured the width of the head between the outer tips of each eye (illustrated in ref. 17) and the distances among three vein intersections on the wing. We estimated size by reducing the wing measurements to a single variable with principal components analysis; the first principal component is generally considered to be an estimate of body size (24). Furthermore, wing vein measurements are highly significantly positively correlated with tibia length in the close relative D. silvestris (25).
To examine the effects of head width on species discrimination, we took advantage of the fact that the reciprocal hybrids show a strong X chromosomal effect on head width (17, 26). Hybrid males with a D. heteroneura mother (referred to as HS below) have broader heads than hybrid males with a D. silvestris mother (SH hybrids) although these reciprocal hybrids have the same autosomal composition. Thus, we tested the hypothesis that, if head width influences species discrimination by females, male D. heteroneura would have the greatest mating success, followed by HS hybrids, followed by SH hybrids. Production of F1 hybrids is described by Price and Boake (19).
For the courtship tests of males with different head widths, we used virgin D. heteroneura females. Each male and each female was tested once, under the same conditions as for the intraspecific tests, except that we watched up to 20 chambers at a time. For the crosses with Y11R6, we tested equal numbers of all three genotypes simultaneously; their relative positions within the array of chambers were randomized. Some of the data from the crosses with U26B9 were reanalyzed from previously published work (H females with H and SH males; ref. 19; Table 2), and some came from a later test (H females with H and HS males). These studies all used the same behavioral methods. The mating success is the proportion of all males of a given genotype (H, HS, SH) that copulated with H females. We also noted the time from the introduction of the female until the first approach, first male wing-vibration, and copulation. Males from the Y11R6 cross were frozen and measured as before; we did not measure males from the U26B9 cross.
We remeasured 24 specimens from the aggression tests to estimate measurement error (27). Repeatabilities of size ranged from 0.8 for measurements of wing veins to 0.9 for head width; the measurement errors were 14% and 13% of the means, respectively. In the tests of species isolation, 10 males of each genotype were measured twice to estimate measurement error; the repeatabilities were 0.99 for head width and 0.95 for size. The measurement errors for head width and size were 3% and 6% of the means, respectively. Because the distributions of the measurements did not differ significantly from normality, statistical tests were conducted on untransformed data.
RESULTS
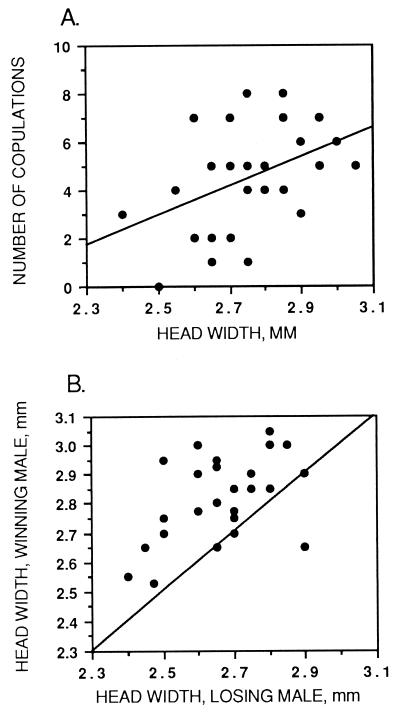
Males with broader heads were significantly more successful in both courtship and aggressive interactions (Fig. 1). The relationship between head width and mating success was more highly significant for a linear fit than for a fit that included a quadratic term for head width. The regression of courtship success on morphology is described by the equation: number of copulations = −35.98 + 7.36 (head width) − 0.81 (wing size), with r2 = 0.28, P = 0.013. The effect of head width was significant [F(1, 27) = 7.59, P = 0.01], but wing size was not significant [F(1, 27) = 3.15, P = 0.09]. The equation for the nonlinear fit is: number of copulations = −79.90 + 2336 (head width) − 1.45 (head width)2 − 0.81 (wing size); r2 = 0.28, P = 0.03. The effects of head width, head width squared, and wing size were not significant in this model [F(1, 26) = 0.49, 0.23, 3.14, P = 0.49, 0.63, 0.09, respectively]. Thus, selection on head width through mate choice appears to be directional rather than stabilizing. In both models, size was negatively associated with mating success.
Figure 1.
Associations between head width and sexually selected behavior within D. heteroneura. (A) Courtship success. The univariate regression line is provided to illustrate the relationship. In this and the next graph, some points represent more than one subject. (B) Aggressive success. The line illustrates equal sizes of winners and losers. In three cases, the head width of the winner and loser were the same. In one case, the winner was smaller than the loser; in this pair, the losing male had a large blob of paint on his eye. Using a likelihood ratio test, the probability of no association between head width and winning fights is 0.007, and the probability of no association between size and winning fights is 0.89.
We found no significant differences in male mating success between the three genotypes in the Y11R6 cross (Table 1); to the extent that the groups differed, HS males had lower mating success than the other two genotypes, which is inconsistent with the directional hypothesis. In the U26B9 crosses, SH males and H males had equal mating success with H females (19), as did HS and H males (16 of 25 tests and 14 of 29 tests, respectively). Contingency table tests for the SH and HS data in the U26B9 crosses showed no significant differences between mating successes of hybrid and H males, with P > 0.2 in each case.
Table 1.
Association between mating success and head width in tests of reciprocal hybrid males
| Male genotype | Number tested | Mating success, % | Head width, mm |
|---|---|---|---|
| D. heteroneura | 78 | 50 | 2.81 ± 0.01 |
| HS hybrid | 79 | 42 | 2.5 ± 0.01 |
| SH hybrid | 75 | 56 | 1.98 ± 0.02 |
The mating successes of the different groups did not differ significantly (homogeneity χ2 = 3.21, df = 2, P > 0.1).
If hybrid males were in fact less attractive, but compensated during courtship, the latency from the first approach until full courtship (wing vibration) should be longer, or the entire courtship should be longer. However, neither the latency from the first approach until wing vibration nor the latency to copulation differed significantly among the three groups of males (Y11R6 cross; Wilcoxon tests, df = 2, P = 0.91 and 0.34, respectively).
DISCUSSION
Our observation that head width in D. heteroneura is sexually selected confirms an untested association that was predicted more than 15 years ago (8). We propose that male flies can easily assess head width during aggressive interactions; a male needs to notice only whether his opponent’s eyes are inside, outside, or at the same distance apart as his own (28). Females could assess male head width while the male is in the frontal position in the early stage of courtship. The negative association between size and male mating success indicates that females are evaluating head width directly rather than using it as an indicator of large body size.
Despite inferences that the two populations of D. silvestris might show different degrees of isolation from D. heteroneura (18), and thus that F1 males from the two crosses might also interact differently with D. heteroneura females, we found the same pattern in each cross: Hybrid males, which had been predicted to show some isolation from D. heteroneura females, showed none. Because of the parallel results from crosses with two morphologically distinct populations of D. silvestris, we are confident that, at best, there is a minor influence of head width on behavioral isolation between the two species. As described below, we still need to search for traits that are involved in species recognition.
Our failure to find behavioral differences between crosses with the two populations of D. silvestris, despite earlier results that suggest that differences should be found (18), could be due to differences in the testing methods: Ahearn and Templeton (18) scored mating success by housing flies together for 4–8 weeks whereas we used 1-h tests. We chose brief tests because, in the forest, unreceptive females can leave at any time; our chambers are large enough to allow unreceptive females to avoid males, as can be seen from the substantial numbers of females that did not mate. Like Ahearn and Templeton (18), we could get larger numbers of H females and S males to mate when they were housed together for a long time, as we show here. We housed 10 virgin females with 10 males in a jar (≈2 liters) for 7 days, then dissected the females to assess insemination (all crosses were between Y11R6 and W48B6). The average proportion of inseminated females out of five replicates was 68% for the H female by S male cross and 88% for the reciprocal cross (Wilcoxon test, P = 0.12). Thus, the degree of isolation that we detected depends on the duration of exposure between females and males, and our results are only strictly comparable to those of tests in which females are given a brief opportunity to interact with males.
Templeton (26) proposed that sexual selection is not important in speciation in the Hawaiian Drosophila because mate choice is stabilizing within species. The sexually selected trait that we examined is not important in behavioral isolation, but the reason is not consistent with Templeton’s proposal because we found directional sexual selection through female mate choice. Our detection of directional selection is not due to sampling a smaller size range than Templeton did because the range of head widths in our study was 2.4–3.1 mm, nearly the same as the range studied by Templeton (17, 26). Statistical analyses of selection are essentially correlational (25, 29), and the cause of selection needs to be confirmed with an experimental approach, such as increasing the male eye span through artificial selection (30). Female preferences for broad heads in distantly related stalk-eyed flies (Diopsidae) also appear to be directional; females preferred to perch near males with heads that had been widened experimentally well beyond the natural range of the species (31).
Our surprising conclusion that male head width plays a minor role, if any, in maintaining behavioral isolation between the species raises two questions. First, what cues are involved in species recognition, and second, why is head width unimportant? To answer the first question, we are currently evaluating the possibilities that chemical or vibrational (21) signals could be cues to species identity. The second question is more difficult. We cannot blame the lack of discrimination on female desperation to mate, because in all our tests, far fewer than 100% of females mated (Fig. 1A and Table 1). Perhaps with a far larger sample size, we would find a significant effect, which is masked because head width explains only a small portion of differential mating success within the species. Another possibility is that head width diverged after reproductive isolation was substantially complete, with divergence of the two populations allowing a change in this secondary sexual character, rather than being driven by it. Finally, the hypothesis that sexual selection and behavioral reproductive isolation form a continuum may be incorrect although it cannot be rejected for this species pair until the other possible sexual signals have been examined.
D. silvestris and D. heteroneura are in an early stage of divergence; the only barriers to hybridization are prezygotic, hybrids are fertile, and only one cross is difficult to produce. The novel sexual dimorphism within D. heteroneura is sexually selected, but it is not involved in isolation between the species. These results are more consistent with Paterson’s hypothesis (5) than with hypotheses that are derived from genetic models, but we have presented data for only one potential cue. A complete test for this species pair will involve evaluating all possible cues that contribute to the initial decision of whether to court; such studies are ongoing. Our method of linking tests of sexual selection and species recognition could be applied to several other species groups for which experimentation is possible. For example, populations and species of green lacewings (Chrysoperla; ref. 32) show behavioral isolation based on courtship song and thus would be excellent for studies of the same song features in sexual selection. Some plethodontid salamanders might also be useful because male chemical signals are thought to affect mate choice within species (33) and may also influence sexual isolation between species (34). Congruent studies on taxonomically diverse groups would provide a substantial body of evidence to evaluate the relationship between sexual selection and species recognition.
Acknowledgments
We thank Gina Baucom, Sarah McKee, Sheryl Moore, and Teresa Roberts for their help with stock keeping. Hamp Carson, Sergey Gavrilets, Alan Templeton, Mike Wade, Jerry Wilkinson, and two anonymous reviewers gave valuable comments on this manuscript. The research was funded with National Science Foundation Grant IBN-9514041.
ABBREVIATIONS
- HS
hybrid males with a D. heteroneura mother
- SH
hybrid males with a D. silvestris mother
References
- 1.Coyne J A. Nature (London) 1992;355:511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- 2.Lande R. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lande R, Kirkpatrick M. J Theor Biol. 1988;133:85–98. doi: 10.1016/s0022-5193(88)80026-2. [DOI] [PubMed] [Google Scholar]
- 4.Turner G F, Burrows M T. Proc R Soc Lond B. 1995;260:287–292. [Google Scholar]
- 5.Paterson H E H. In: Species and Speciation, Transvaal Museum Monograph No. 4. Vrba E S, editor. Pretoria, South Africa: Transvaal Museum; 1985. pp. 21–29. [Google Scholar]
- 6.Butlin R. In: Genetic Variation in Mating Signals and Responses. Lambert D M, Spencer H G, editors. Baltimore: Johns Hopkins Univ. Press; 1995. pp. 327–366. [Google Scholar]
- 7.Carson H L. In: Evolutionary Processes and Theory. Karlin S, Nevo E, editors. New York: Academic; 1986. pp. 93–107. [Google Scholar]
- 8.Spieth H T. Evolution. 1981;35:921–930. doi: 10.1111/j.1558-5646.1981.tb04958.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaneshiro K Y, Val F C. Am Nat. 1977;111:897–902. [Google Scholar]
- 10.Carson H L, Kaneshiro K Y, Val F C. Evolution. 1989;43:190–203. doi: 10.1111/j.1558-5646.1989.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 11.Bradbury J W. In: Experimental Behavioral Ecology. Hölldobler B, Lindauer M, editors. New York: G. Fischer Verlag; 1985. pp. 273–289. [Google Scholar]
- 12.Templeton A R. Evolution. 1979;33:513–517. doi: 10.1111/j.1558-5646.1979.tb04704.x. [DOI] [PubMed] [Google Scholar]
- 13.Hedrick P W. Genetics of Populations. Boston: Jones & Bartlett; 1985. pp. 492–493. [Google Scholar]
- 14.Futuyma D J. Evolutionary Biology. 2nd ed. Sunderland, MA: Sinauer; 1986. p. 221. [Google Scholar]
- 15.Maynard Smith J. Evolutionary Genetics. Oxford: Oxford Univ. Press; 1989. p. 278. [Google Scholar]
- 16.Craddock E M. Evolution. 1974;28:593–606. doi: 10.1111/j.1558-5646.1974.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 17.Val F C. Evolution. 1977;31:611–629. doi: 10.1111/j.1558-5646.1977.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahearn J N, Templeton A R. Evolution. 1989;43:347–361. doi: 10.1111/j.1558-5646.1989.tb04232.x. [DOI] [PubMed] [Google Scholar]
- 19.Price D K, Boake C R B. J Insect Behav. 1995;8:595–616. [Google Scholar]
- 20.Fraser I, Boake C R B. Am Nat. 1997;149:527–539. [Google Scholar]
- 21.Hoikkala A, Welbergen P. Anim Behav. 1995;50:177–190. [Google Scholar]
- 22.Carson H L, Val F C, Simon C M, Archie J W. Evolution. 1982;36:132–140. doi: 10.1111/j.1558-5646.1982.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaneshiro K Y, Kurihara J S. Pac Sci. 1981;35:177–183. [Google Scholar]
- 24.Reyment R A, Blacklith R E, Campbell N A. Multivariate Morphometrics. New York: Academic; 1984. [Google Scholar]
- 25.Boake C R B. Ethology. 1989;80:318–329. [Google Scholar]
- 26.Templeton A R. Evolution. 1977;31:530–541. doi: 10.1111/j.1558-5646.1977.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 27.Lessels C M, Boag P T. Auk. 1987;104:116–121. [Google Scholar]
- 28.McAlpine D K. In: Sexual Selection and Reproductive Competition in Insects. Blum M S, Blum N A, editors. New York: Academic; 1979. pp. 221–230. [Google Scholar]
- 29.Wade M J, Kalisz S. Evolution. 1990;44:1947–1955. doi: 10.1111/j.1558-5646.1990.tb04301.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson G S, Reillo P R. Proc R Soc Lond B. 1994;255:1–6. [Google Scholar]
- 31.Burkhardt D, de la Motte I. J Comp Physiol A. 1988;162:649–652. [Google Scholar]
- 32.Wells M M, Henry C S. Evolution. 1992;46:31–42. doi: 10.1111/j.1558-5646.1992.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 33.Houck L D, Reagan N L. Anim Behav. 1990;39:729–734. [Google Scholar]
- 34.Arnold S J, Reagan N L, Verrell P A. Herpetologica. 1993;49:216–228. [Google Scholar]