Abstract
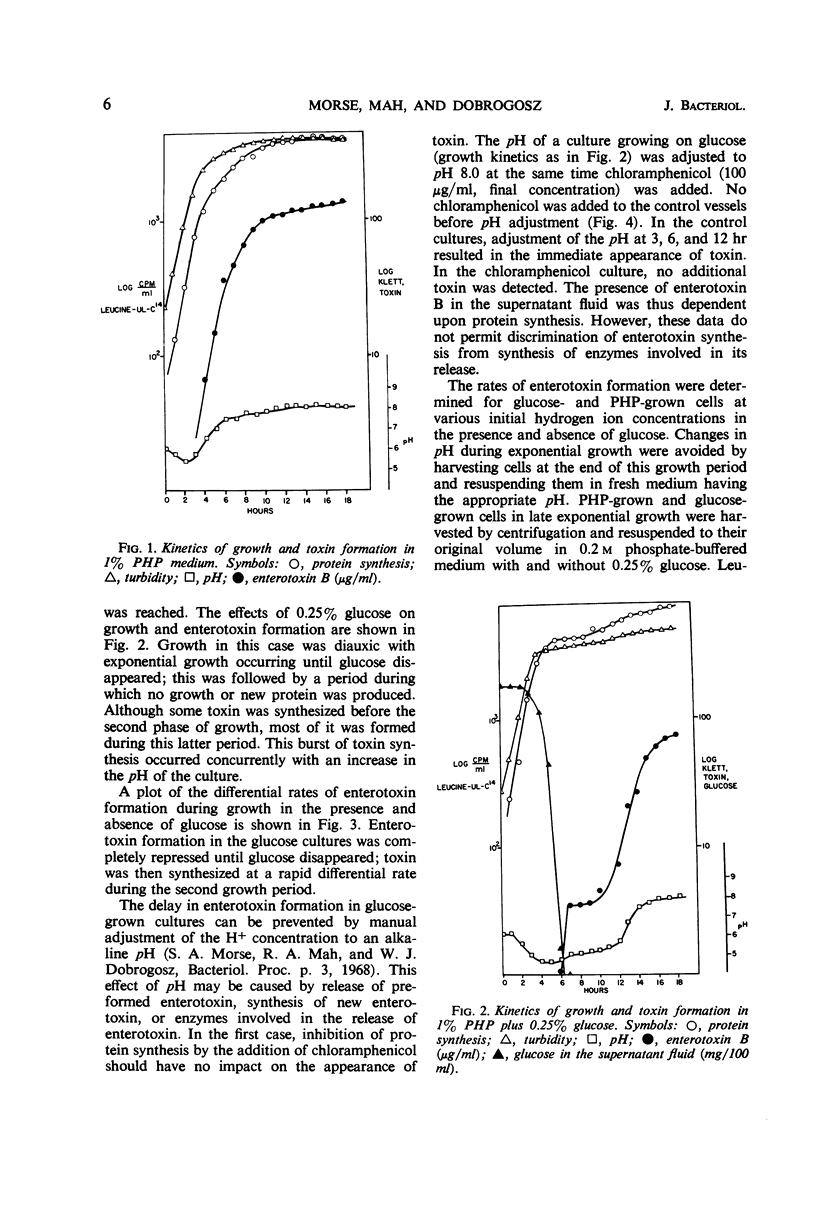
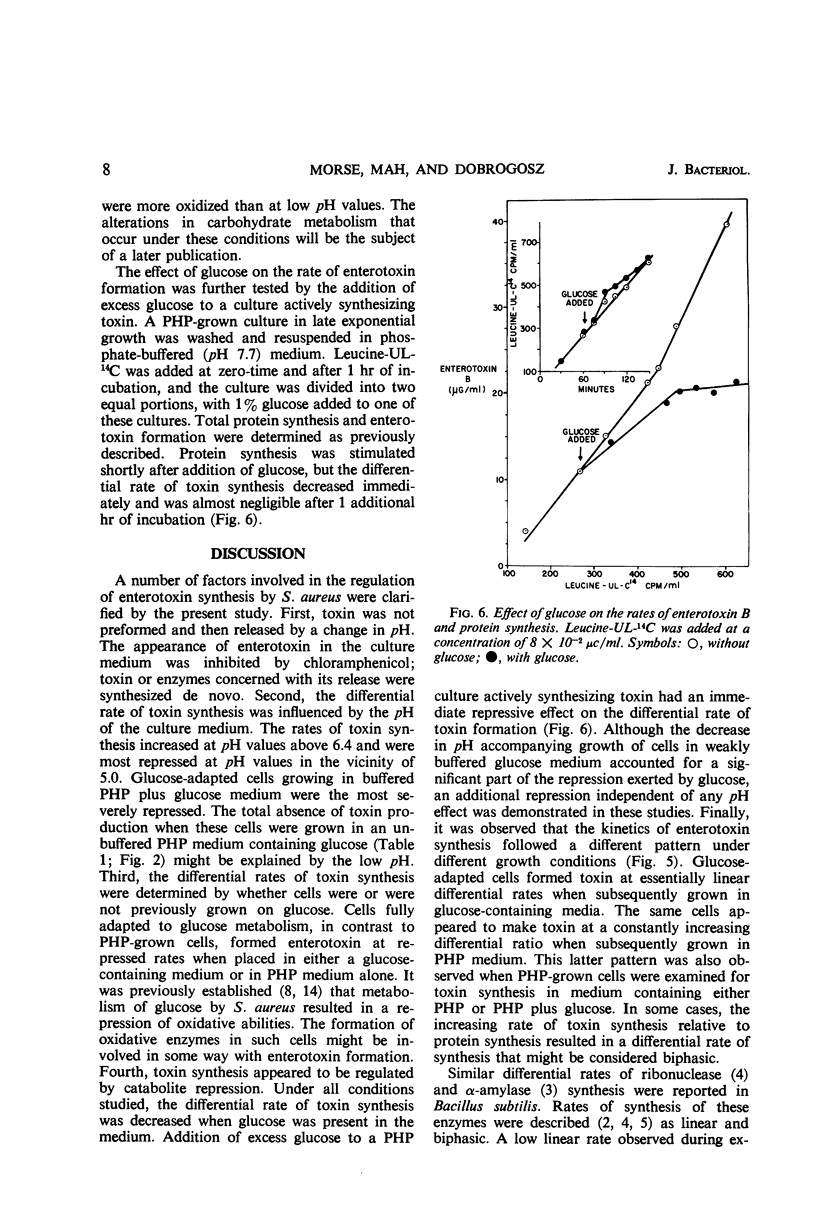
Several factors influenced the formation of enterotoxin B by Staphylococcus aureus strain S-6. In the standard casein hydrolysate medium, toxin was not produced in detectable quantities during exponential growth; it was produced during the post-exponential phase when total protein synthesis was arithmetic. The rate of toxin synthesis was much greater than the rate of total protein synthesis. The appearance of enterotoxin was inhibited by chloramphenicol; thus, the presence of toxin was dependent on de novo protein synthesis. When low concentrations of glucose (<0.30%) were added to the casein hydrolysate medium, growth was diauxic; glucose was completely metabolized during the first growth period. During the second growth period, enterotoxin was synthesized. In unbuffered casein hydrolysate medium containing excess glucose, toxin synthesis was completely repressed. The absence of toxin production under such conditions might be explained by the low (4.6) pH resulting from the acid end products of glucose metabolism. At pH <5.0, little or no toxin was produced. Toxin synthesis was initiated in the presence of glucose when the medium were buffered at any pH above 5.6. In such media, the differential rates of toxin synthesis, with respect to the rates of total protein synthesis, were lower than the differential rates in casein hydrolysate medium alone. Addition of glucose to a culture synthesizing toxin resulted in an immediate decrease in the differential rate without any change in pH. Thus, toxin synthesis appeared to be regulated by catabolite repression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASMAN E. P. Serologic studies of staphylococcal enterotoxin. Public Health Rep. 1958 Jul;73(7):599–609. [PMC free article] [PubMed] [Google Scholar]
- COLEMAN G., ELLIOTT W. H. EXTRACELLULAR RIBONUCLEASE FORMATION IN BACILLUS SUBTILIS AND ITS STIMULATION BY ACTINOMYCIN D. Biochem J. 1965 Jun;95:699–706. doi: 10.1042/bj0950699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN G., ELLIOTT W. H. Studies on alpha-amylase formation by Bacillus subtilis. Biochem J. 1962 May;83:256–263. doi: 10.1042/bj0830256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman G., Grant M. A. Characteristics of alpha-amylase formation by Bacillus subtilis. Nature. 1966 Jul 16;211(5046):306–307. doi: 10.1038/211306a0. [DOI] [PubMed] [Google Scholar]
- Favorite G. O., Hammon W. M. The Production of Staphylococcus Enterotoxin and Alpha Hemolysin in a Simplified Medium. J Bacteriol. 1941 Mar;41(3):305–316. doi: 10.1128/jb.41.3.305-316.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivler D. Comparative metabolism of virulent and avirulent staphylococci. Ann N Y Acad Sci. 1965 Jul 23;128(1):62–80. doi: 10.1111/j.1749-6632.1965.tb11630.x. [DOI] [PubMed] [Google Scholar]
- MANGALO R., PILLET J., RAYNAUD M. Etudes sur la toxine alpha de Staphylococcus pyogenes. Rapports entre croissance et toxinogénese. C R Hebd Seances Acad Sci. 1954 May 10;238(19):1931–1933. [PubMed] [Google Scholar]
- Mah R. A., Fung D. Y., Morse S. A. Nutritional requirements of Staphylococcus aureus S-6. Appl Microbiol. 1967 Jul;15(4):866–870. doi: 10.1128/am.15.4.866-870.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus Z., Silverman G. J. Enterotoxin B production by nongrowing cells of Staphylococcus aureus. J Bacteriol. 1968 Oct;96(4):1446–1447. doi: 10.1128/jb.96.4.1446-1447.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. A., Lilly H. D., Alford J. A. Effects of meat-curing salts and temperature on production of staphylococcal enterotoxin B. J Bacteriol. 1968 Apr;95(4):1207–1211. doi: 10.1128/jb.95.4.1207-1211.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASTERS K. C., WINKLER K. C. CARBOHYDRATE METABOLISM OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Nov;33:213–229. doi: 10.1099/00221287-33-2-213. [DOI] [PubMed] [Google Scholar]
- Weirether F. J., Lewis E. E., Rosenwald A. J., Lincoln R. E. Rapid quantitative serological assay of staphylococcal enterotoxin B. Appl Microbiol. 1966 Mar;14(2):284–291. doi: 10.1128/am.14.2.284-291.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


