Abstract
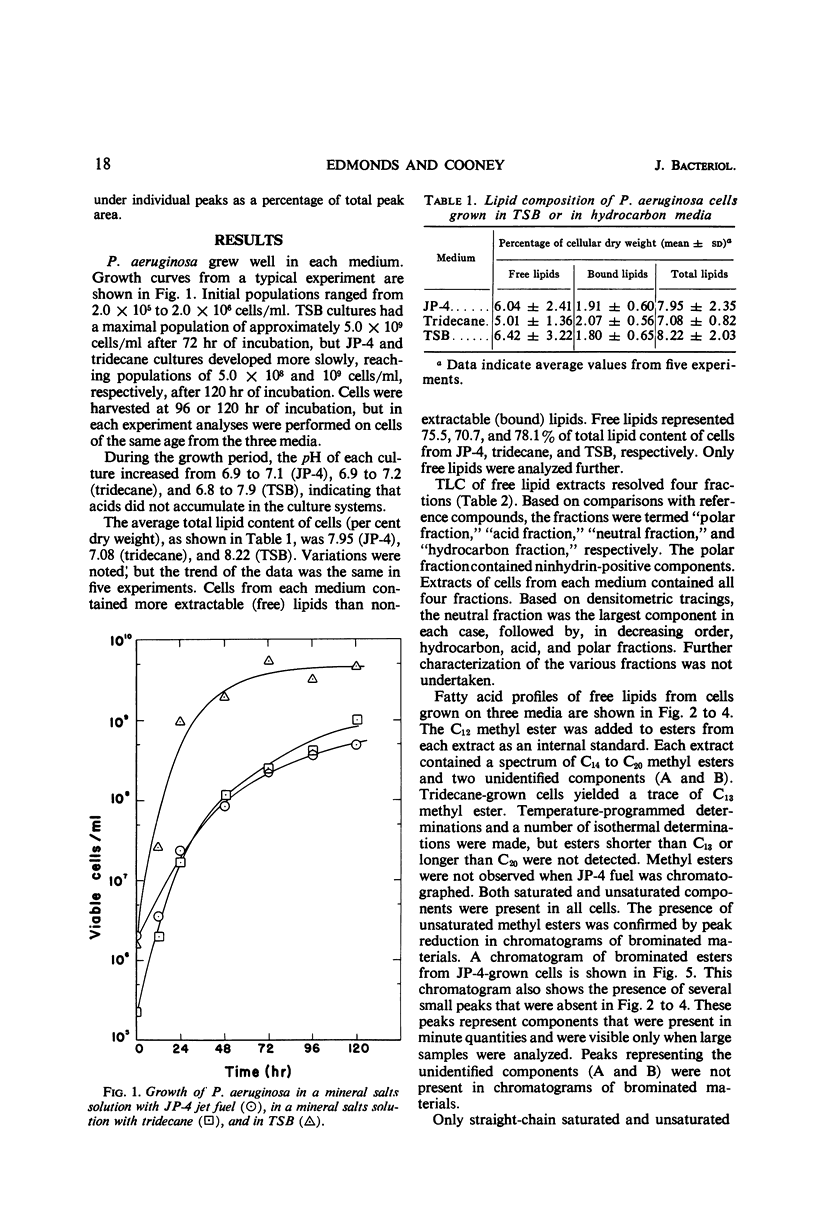
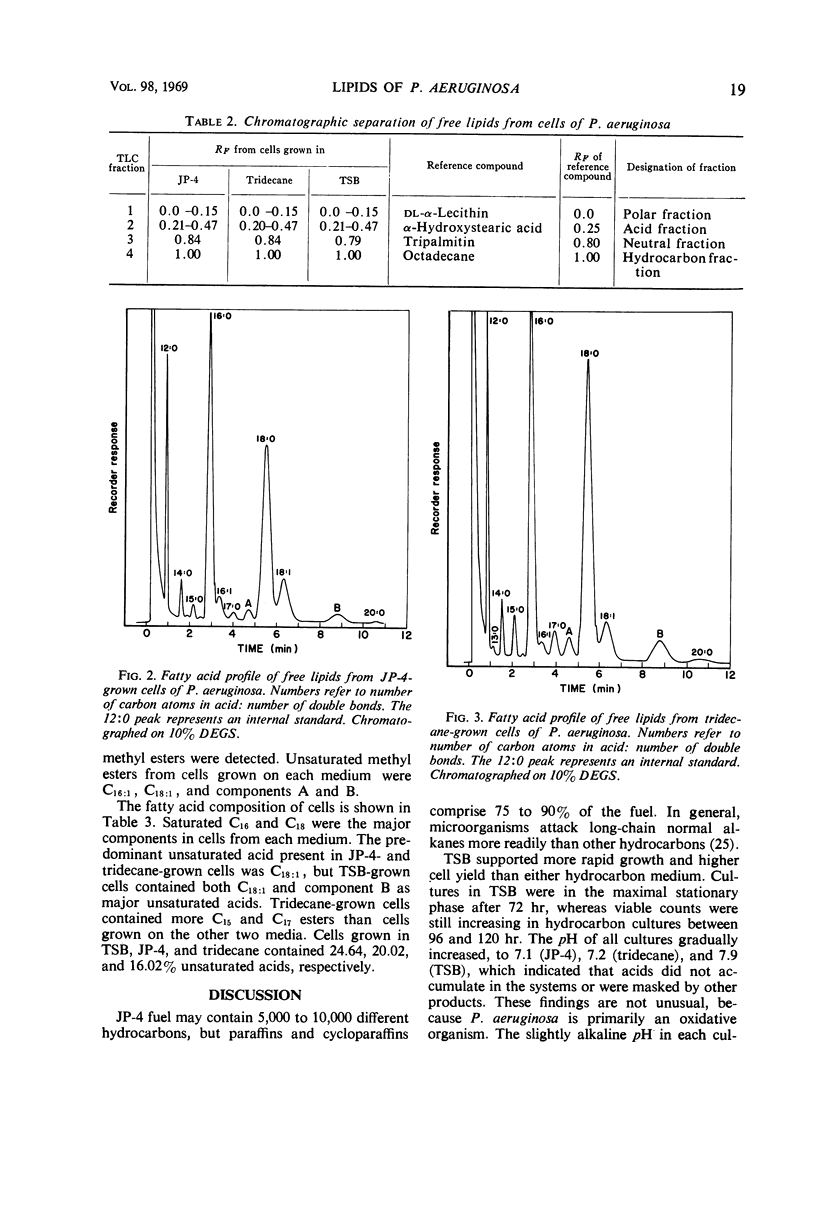
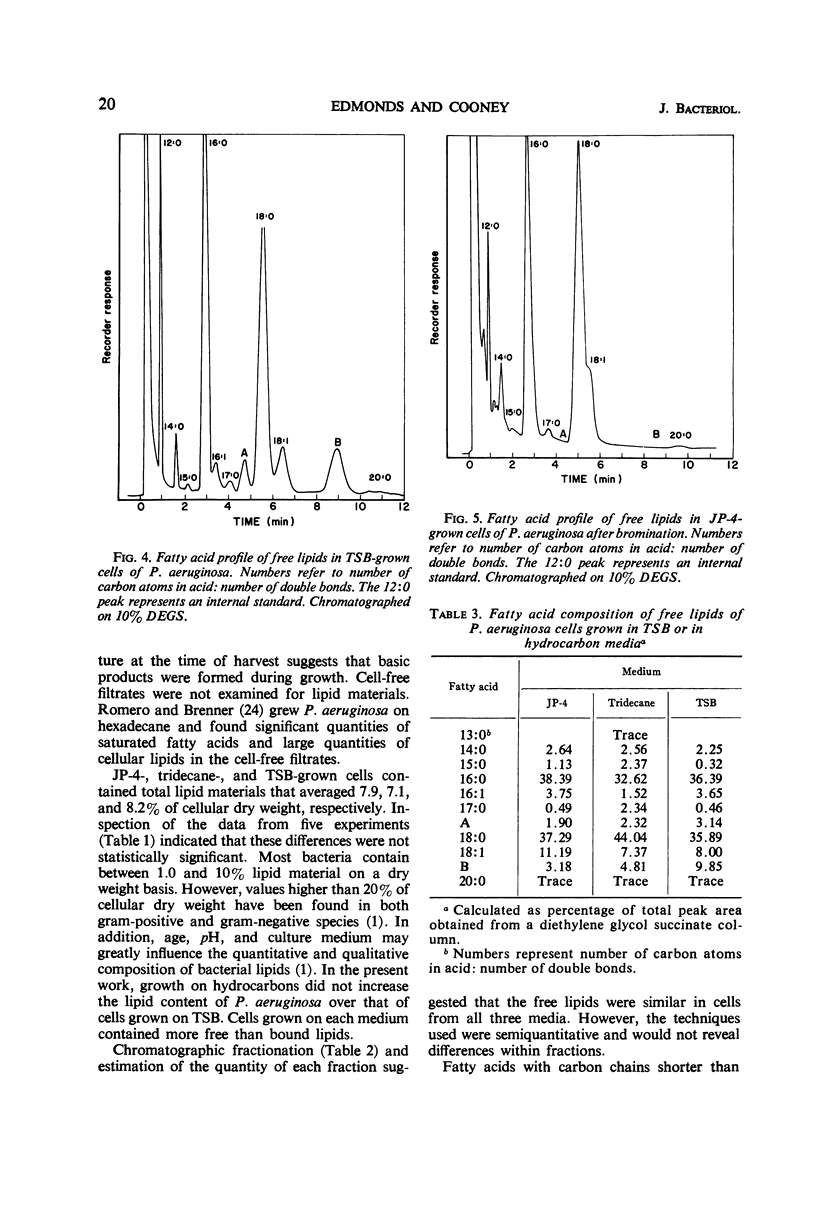
Lipids were extracted from cells of Pseudomonas aeruginosa grown on a pure hydrocarbon (tridecane), mixed hydrocarbons (JP-4 jet fuel), and on Trypticase Soy Broth. Total lipids produced from each substrate represented from 7.1 to 8.2% of cellular dry weight, of which 5.0 to 6.4% were obtained before cellular hydrolysis (free lipids) and 1.7 to 2.0% were extracted after cellular hydrolysis (bound lipids). Free lipids from cells grown on each medium were separated into four fractions by thin-layer chromatography. All fractions were present in cells from each type of medium, and the “neutral fraction” constituted the largest fraction. The fatty acid composition of free lipids was determined by gas-liquid chromatography. Cells grown on each medium contained saturated and unsaturated C14 to C20 fatty acids. Trace amounts of C13 fatty acids were found in tridecane-grown cells. Saturated C16 and C18 were the major acids present in all cells. Quantitative differences were found in fatty acids produced on the three media, but specific correlations between substrate carbon sources and fatty acid content of cells were not evident. Tridecane-grown cells contained only traces of C13 acid and small amounts of C15 and C17 acids, suggesting that the organism's fatty acids were derived from de novo synthesis rather than by direct incorporation of the hydrocarbon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bushnell L. D., Haas H. F. The Utilization of Certain Hydrocarbons by Microorganisms. J Bacteriol. 1941 May;41(5):653–673. doi: 10.1128/jb.41.5.653-673.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS J. B. MICROBIAL INCORPORATION OF FATTY ACIDS DERIVED FROM N-ALKANES INTO GLYCERIDES AND WAXES. Appl Microbiol. 1964 May;12:210–214. doi: 10.1128/am.12.3.210-214.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of Substrate on the Fatty Acid Composition of Hydrocarbon- and Ketone-utilizing Microorganisms. J Bacteriol. 1968 Aug;96(2):318–321. doi: 10.1128/jb.96.2.318-321.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of substrate on the fatty acid composition of hydrocabon-utilizing microorganisms. J Bacteriol. 1967 Dec;94(6):1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds P. Selection of test organisms for use in evaluating microbial inhibitors in fuel-water systems. Appl Microbiol. 1965 Sep;13(5):823–824. doi: 10.1128/am.13.5.823-824.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney F. W., Markovetz A. J., Kallio R. E. Bacterial oxidation of 2-tridecanone to 1-undecanol. J Bacteriol. 1967 Feb;93(2):649–655. doi: 10.1128/jb.93.2.649-655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks K. M. Products of the oxidation of n-decane by Pseudomonas aeruginosa and Mycobacterium rhodochrous. Antonie Van Leeuwenhoek. 1967;33(1):41–48. doi: 10.1007/BF02045532. [DOI] [PubMed] [Google Scholar]
- HORNING E. C., AHRENS E. H., Jr, LIPSKY S. R., MATTSON F. H., MEAD J. F., TURNER D. A., GOLDWATER W. H. QUANTITATIVE ANALYSIS OF FATTY ACIDS BY GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1964 Jan;5:20–27. [PubMed] [Google Scholar]
- HUSTON C. K., ALBRO P. W. LIPIDS OF SARCINA LUTEA. I. FATTY ACID COMPOSITION OF THE EXTRACTABLE LIPIDS. J Bacteriol. 1964 Aug;88:425–432. doi: 10.1128/jb.88.2.425-432.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T., MARTIN A. J. Gas-liquid chromatography: the separation and identification of the methyl esters of saturated and unsaturated acids from formic acid to n-octadecanoic acid. Biochem J. 1956 May;63(1):144–152. doi: 10.1042/bj0630144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA T. Biosythesis of branched chain fatty acids. I. Isolation and identification of fatty acids from Bacillus subtilis (ATCC 7059). J Biol Chem. 1963 Apr;238:1222–1228. [PubMed] [Google Scholar]
- KATES M., KUSHNER D. J., JAMES A. T. The lipid composition of Bacillus cereus as influenced by the presence of alcohols in the culture medium. Can J Biochem Physiol. 1962 Jan;40:83–94. [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J Bacteriol. 1967 Mar;93(3):894–903. doi: 10.1128/jb.93.3.894-903.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Degradation of hydrocarbons by members of the genus Candida. II. Oxidation of n-alkanes and l-alkenes by Candida lipolytica. J Bacteriol. 1967 Jun;93(6):1847–1852. doi: 10.1128/jb.93.6.1847-1852.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Bacterial oxidation of gaseous alkanes. Arch Mikrobiol. 1960;35:92–104. doi: 10.1007/BF00425597. [DOI] [PubMed] [Google Scholar]
- Makula R., Finnerty W. R. Microbial assimilation of hydrocarbons. I. Fatty acids derived from normal alkanes. J Bacteriol. 1968 Jun;95(6):2102–2107. doi: 10.1128/jb.95.6.2102-2107.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'leary W. M. THE FATTY ACIDS OF BACTERIA. Bacteriol Rev. 1962 Dec;26(4):421–447. doi: 10.1128/br.26.4.421-447.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. E., Berger L. R. Complex lipids of Rhodomicrobium vannielii. J Bacteriol. 1967 Jan;93(1):221–229. doi: 10.1128/jb.93.1.221-229.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. E., Berger L. R. Fatty acids of extractable and bound lipids of Rhodomicrobium vannielii. J Bacteriol. 1967 Jan;93(1):230–236. doi: 10.1128/jb.93.1.230-236.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene T. G., Bennett E. O., Oró J. Fatty acid and aliphatic hydrocarbon composition of Sarcina lutea grown in three different media. J Bacteriol. 1967 Aug;94(2):344–348. doi: 10.1002/path.1700940212. [DOI] [PMC free article] [PubMed] [Google Scholar]



