Abstract
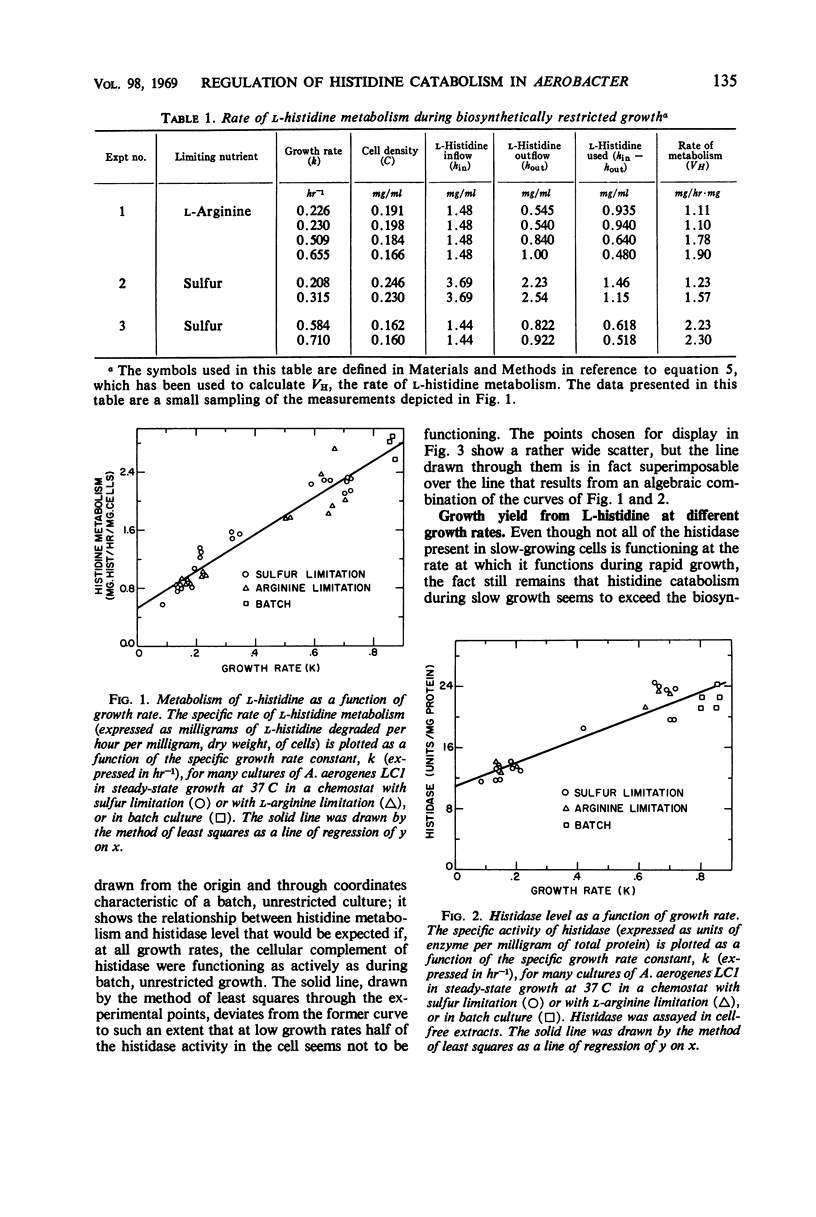
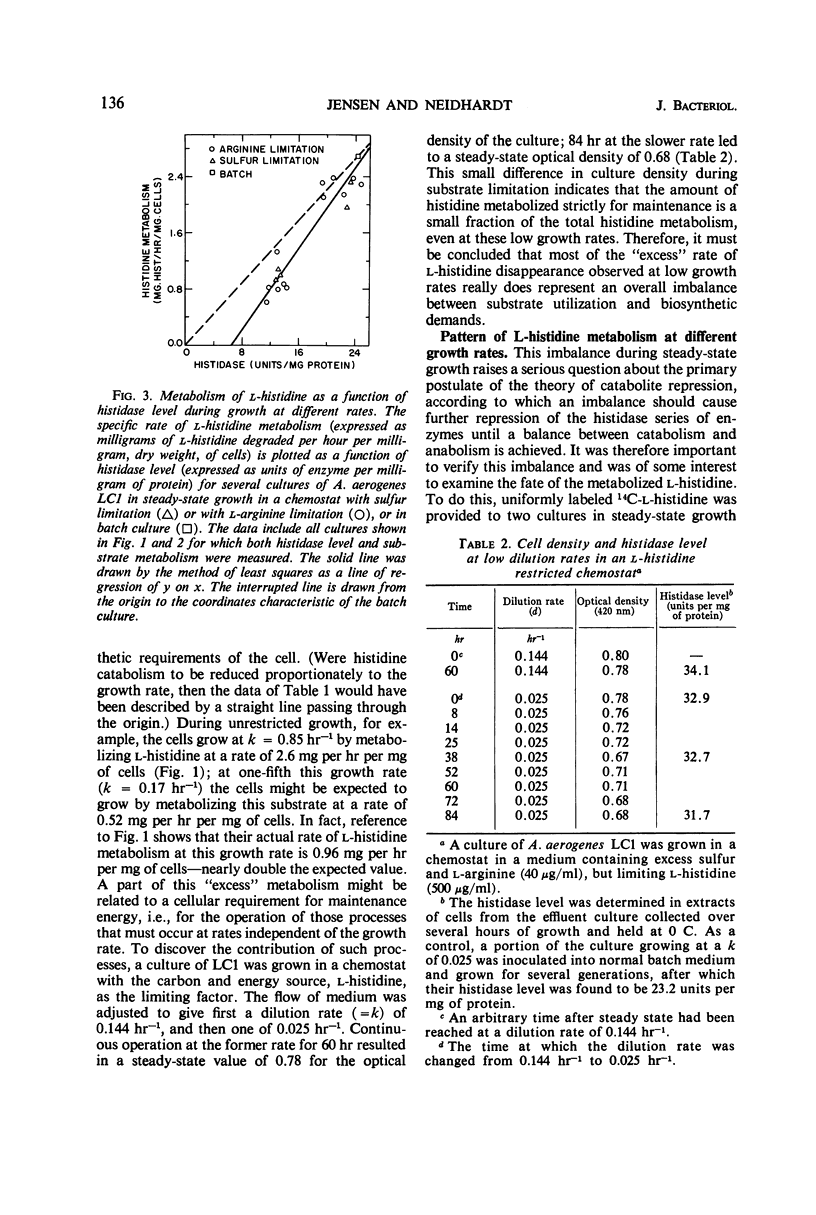
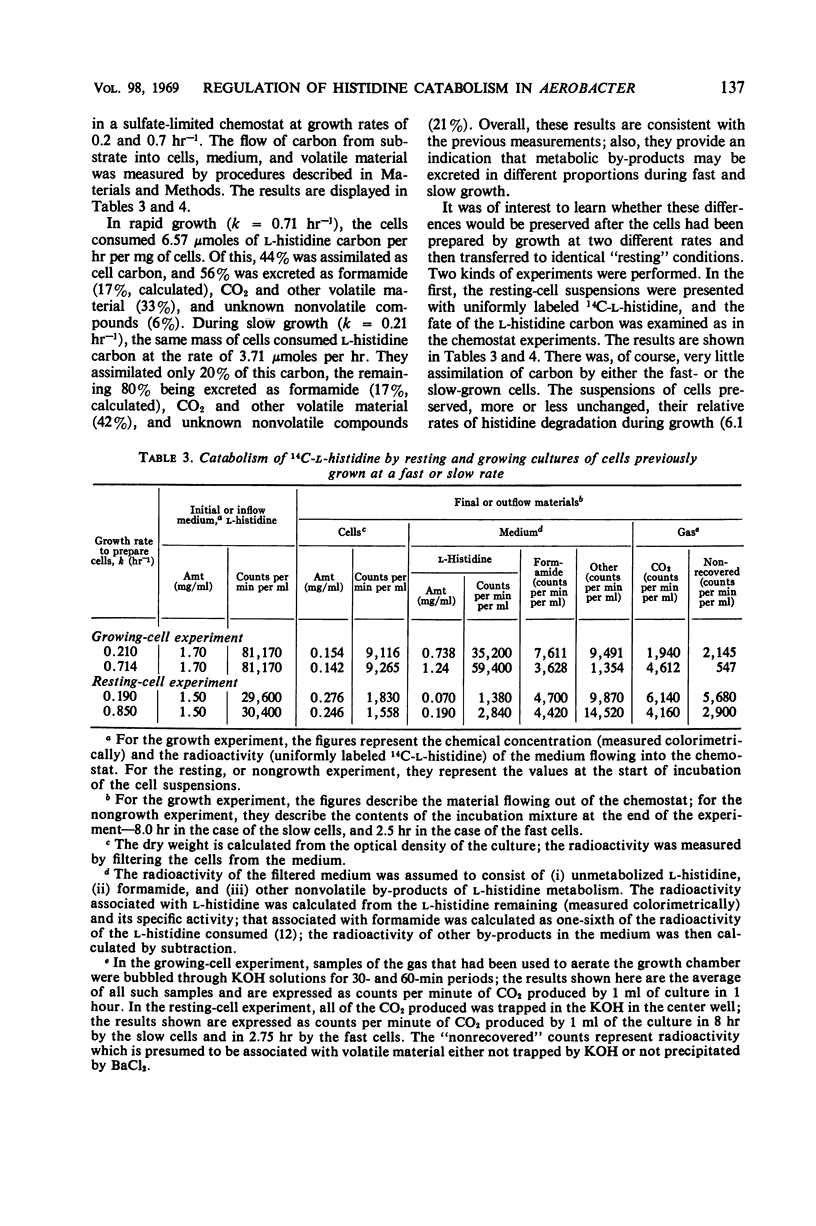
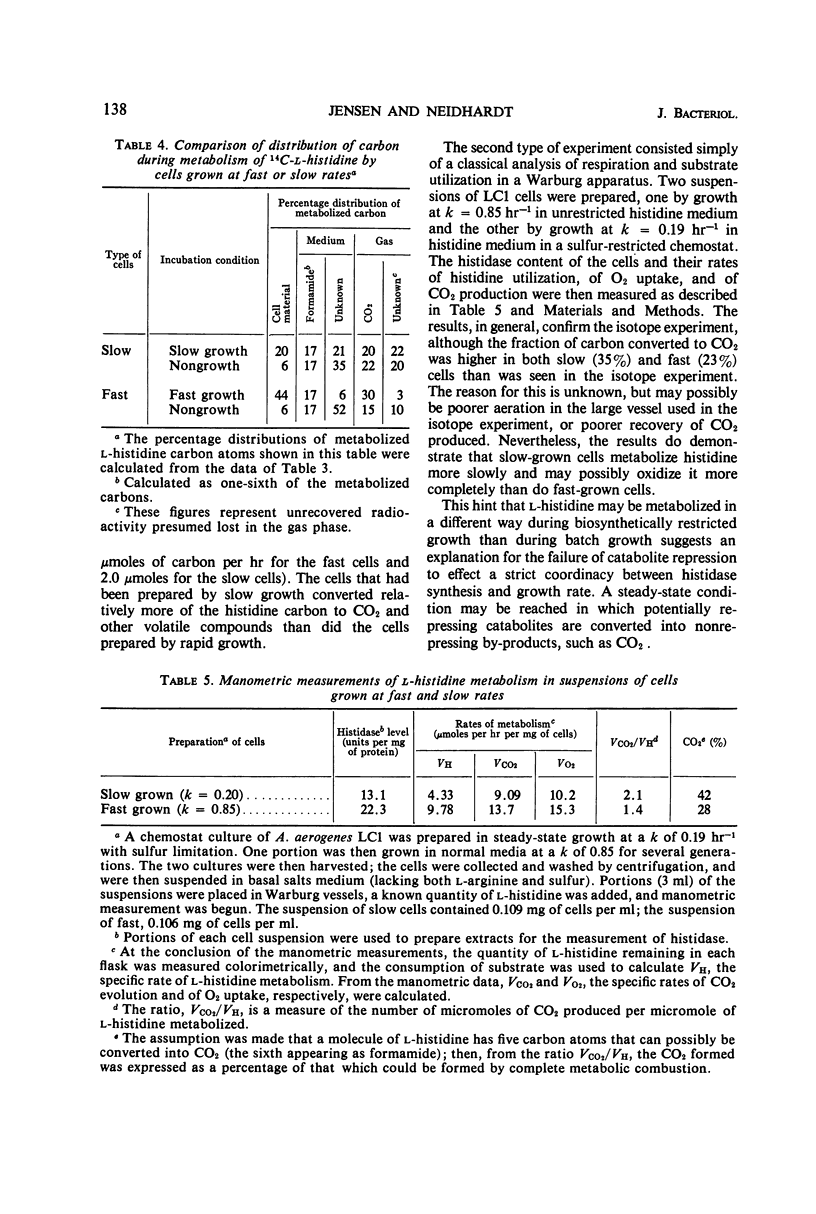
A study was made of how the catabolism of a carbon and energy source is affected by the biosynthetic demands of growing bacterial cells. Cultures of Aerobacter aerogenes in l-histidine medium were grown in a chemostat at rates determined by the supply of either sulfate or a required amino acid, l-arginine. It was discovered that the rate at which these cells grow under a biosynthetic restriction determines both the rate and the pattern of histidine degradation. (i) Histidine catabolism is partially coupled to the growth rate. This coupling is achieved by catabolite repression of histidase (histidine ammonia lyase; EC 4.3.1.3.), and also by a slightly decreased in vivo function of this enzyme at low growth rates. (ii) The looseness of the coupling results in a direct relationship between growth rate and growth yield, and possibly is correlated with an altered pattern of carbon flow from histidine. (iii) Sudden decreases in growth rate cause total repression of histidase synthesis for substantial periods of time. (iv) Sudden release of biosynthetic restriction leads rapidly to an increase in the functioning of the cells' complement of histidase, an increase in the rate of synthesis of this enzyme, and an increase in the growth yield from histidine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daesch G., Mortenson L. E. Sucrose catabolism in Clostridium pasteurianum and its relation to N2 fixation. J Bacteriol. 1968 Aug;96(2):346–351. doi: 10.1128/jb.96.2.346-351.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug D. H., Roth D., Hunter J. Regulation of histidine catabolism by succinate in Pseudomonas putida. J Bacteriol. 1968 Aug;96(2):396–402. doi: 10.1128/jb.96.2.396-402.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorpes E. The colorimetric determination of histidine. Biochem J. 1932;26(5):1507–1511. doi: 10.1042/bj0261507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE GALL J., SENEZ J. C. [Influence of nitrogen fixation on the growth of Desulfovibrio desulfuricans]. C R Hebd Seances Acad Sci. 1960 Jan 11;250:404–406. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Magasanik B., Lund P., Neidhardt F. C., Schwartz D. T. Induction and repression of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4320–4324. [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Reversal of the glucose inhibition of histidase biosynthesis in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):253–259. doi: 10.1128/jb.73.2.253-259.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- SENEZ J. C. Some considerations on the energetics of bacterial growth. Bacteriol Rev. 1962 Jun;26:95–107. [PMC free article] [PubMed] [Google Scholar]


