Abstract
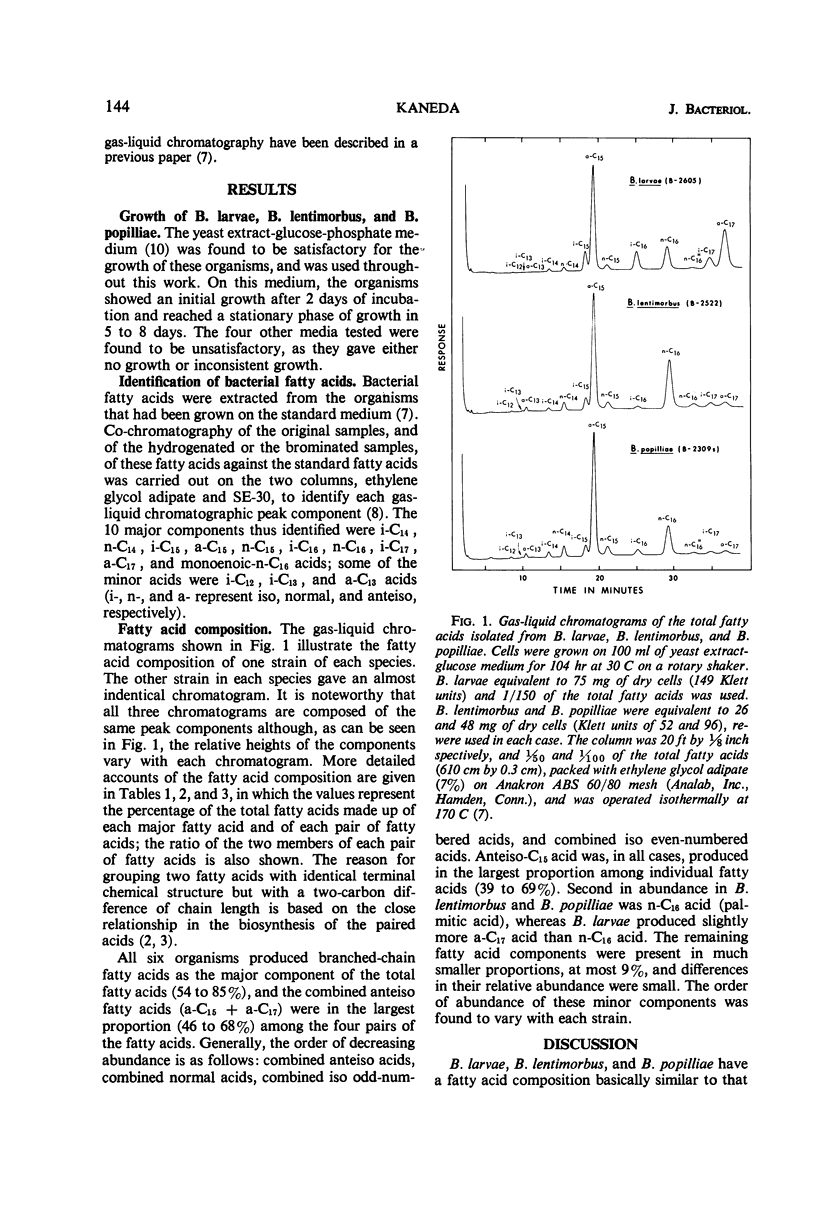
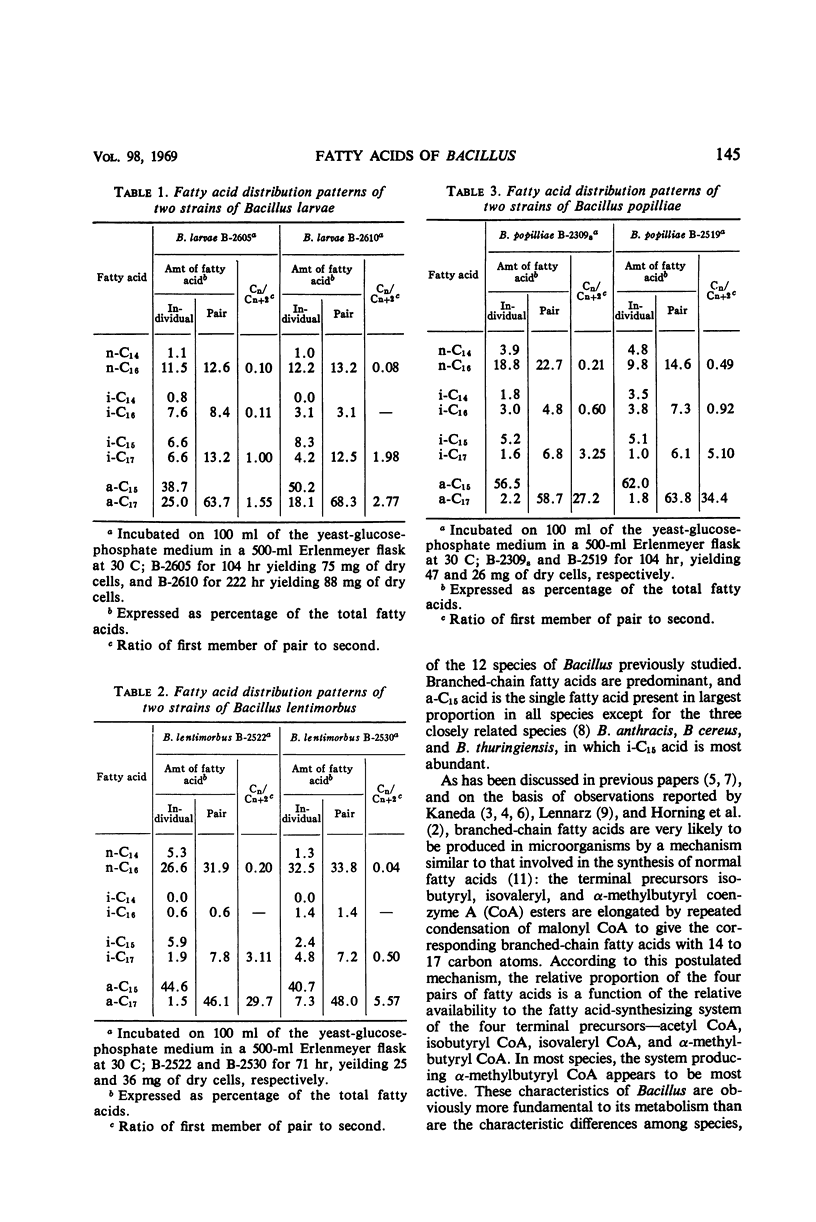
The types of fatty acids produced by two strains each of Bacillus larvae, B. lentimorbus, and B. popilliae, and their distribution patterns, were studied by gas-liquid chromatography. All six organisms produced eight major fatty acids: six branched (iso-C14, -C15, -C16, and -C17, and anteiso-C15 and -C17), two normal (n-C14 and -C16), and two minor (n-C15 and monounsaturated n-C16). In addition, some other trace acids were produced. Branched-chain fatty acids accounted for 54 to 85% of the total fatty acids. These compositions are similar to those previously found with 26 strains of 12 species of the genus Bacillus. Thus, an abundance of branched-chain fatty acids seems to be a characteristic of the biochemical nature of the genus Bacillus. It is noteworthy that marked differences between the nutritional requirements of the three insect pathogens used in the present study and those of the other 12 species of the genus Bacillus studied previously are not significantly reflected in their fatty acid composition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HORNING M. G., MARTIN D. B., KARMEN A., VAGELOS P. R. Fatty acid synthesis in adipose tissue. II. Enzymatic synthesis of branched chain and odd-numbered fatty acids. J Biol Chem. 1961 Mar;236:669–672. [PubMed] [Google Scholar]
- KANEDA T. Biosynthesis of branched chain fatty acids. II. Microbial synthesis of branched long chain fatty acids from certain short chain fatty acid substrates. J Biol Chem. 1963 Apr;238:1229–1235. [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. V. Microbial stereospecific syntheses of D-12-methyltetradecanoic and D-14-methylhexadecanoic acids. Biochim Biophys Acta. 1966 Aug 3;125(1):43–54. doi: 10.1016/0005-2760(66)90142-1. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J Bacteriol. 1967 Mar;93(3):894–903. doi: 10.1128/jb.93.3.894-903.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. II. Similarity in the fatty acid compositions of Bacillus thuringiensis, Bacillus anthracis, and Bacillus cereus. J Bacteriol. 1968 Jun;95(6):2210–2216. doi: 10.1128/jb.95.6.2210-2216.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNARZ W. J. The role of isoleucine in the biosynthesis of branched-chain fatty acids by Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1961 Nov 1;6:112–116. doi: 10.1016/0006-291x(61)90395-3. [DOI] [PubMed] [Google Scholar]
- SYLVESTER C. J., COSTILOW R. N. NUTRITIONAL REQUIREMENTS OF BACILLUS POPILLIAE. J Bacteriol. 1964 Jan;87:114–119. doi: 10.1128/jb.87.1.114-119.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIL S. J. Lipid metabolism. Annu Rev Biochem. 1962;31:369–406. doi: 10.1146/annurev.bi.31.070162.002101. [DOI] [PubMed] [Google Scholar]


