Abstract
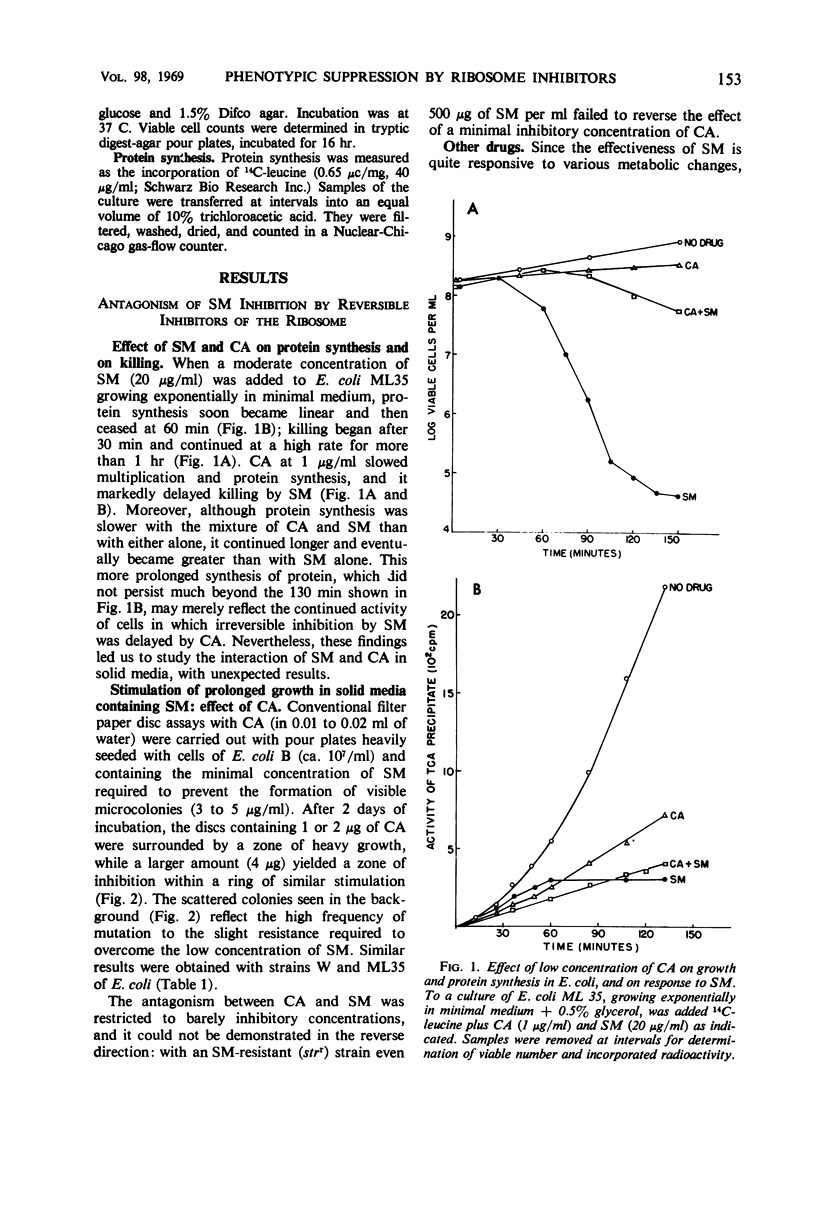
Antibiotics that interfere reversibly with various aspects of ribosomal function (chloramphenicol, tetracycline, erythromycin, and spectinomycin) are shown to antagonize, at barely inhibitory concentrations, the inhibitory effect of low concentrations of streptomycin (SM) on the growth of Escherichia coli. Paradoxically, these compounds can also replace SM in supporting the growth of conditionally SM-dependent mutants. Chloramphenicol produced about as much phenotypic suppression as SM in SM-sensitive strains, but less than that attainable with high concentrations of SM in resistant strains. The antagonism to SM inhibition and the phenotypic suppression appear to be specific for those growth inhibitors that act on the ribosome. Since inhibitors of the 50S subunit of the ribosome (chloramphenicol, erythromycin) are as active as inhibitors of the 30S subunit, it is suggested that phenotypic suppression by borderline concentrations of ribosome inhibitors does not necessarily depend on an alteration of the recognition region of the ribosome. Alternatively, partial inhibition of the ribosomes might change the environment in a way that would influence the frequency of misreading. Phenotypic suppression by a low concentration of SM as well as by chloramphenicol was found to depend on the presence of a trace of the required growth factor.
Full text
PDF

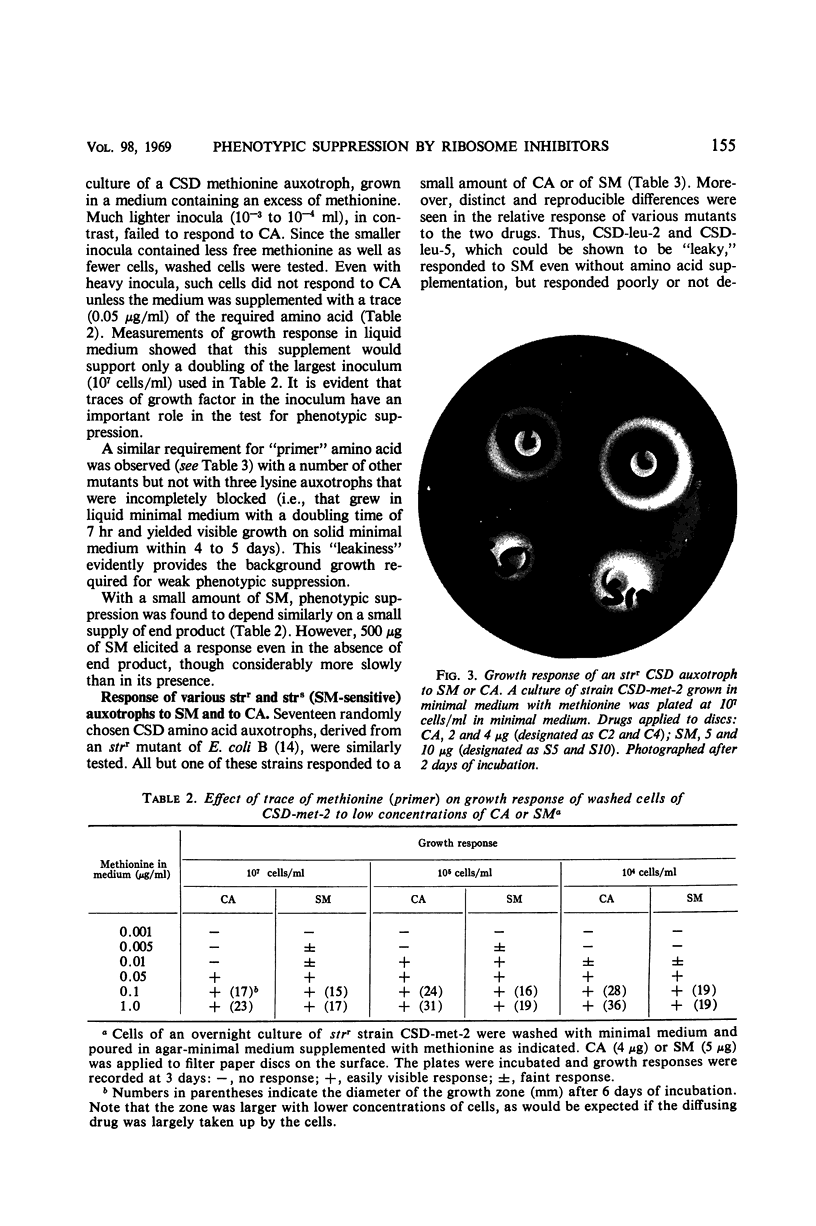


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D. Damage by streptomycin to the cell membrane of Escherichia coli. Nature. 1960 Jan 2;185:22–23. doi: 10.1038/185022a0. [DOI] [PubMed] [Google Scholar]
- Anderson P., Davies J., Davis B. D. Effect of spectinomycin on polypeptide synthesis in extracts of Escherichia coli. J Mol Biol. 1967 Oct 14;29(1):203–215. doi: 10.1016/0022-2836(67)90191-x. [DOI] [PubMed] [Google Scholar]
- Bondi A., Jr, Dietz C. C., Spaulding E. H. Interference With the Antibacterial Action of Streptomycin by Reducing Agents. Science. 1946 Mar 29;103(2674):399–401. doi: 10.1126/science.103.2674.399. [DOI] [PubMed] [Google Scholar]
- CONNAMACHER R. H., MANDEL H. G. BINDING OF TETRACYCLINE TO THE 30S RIBOSOMES AND TO POLYURIDYLIC ACID. Biochem Biophys Res Commun. 1965 Jun 18;20:98–103. doi: 10.1016/0006-291x(65)90954-x. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Anderson P., Davis B. D. Inhibition of protein synthesis by spectinomycin. Science. 1965 Sep 3;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- Day L. E. Tetracycline inhibition of cell-free protein synthesis. I. Binding of tetracycline to components of the system. J Bacteriol. 1966 May;91(5):1917–1923. doi: 10.1128/jb.91.5.1917-1923.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day L. E. Tetracycline inhibition of cell-free protein synthesis. II. Effect of the binding of tetracycline to the components of the system. J Bacteriol. 1966 Jul;92(1):197–203. doi: 10.1128/jb.92.1.197-203.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. PHENOTYPIC REPAIR BY STREPTOMYCIN OF DEFECTIVE GENOTYPES IN E. COLI. Proc Natl Acad Sci U S A. 1964 Mar;51:487–493. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. STREPTOMYCIN-INDUCED OVERSUPPRESSION IN E. COLI. Proc Natl Acad Sci U S A. 1964 Jun;51:995–1001. doi: 10.1073/pnas.51.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L., Jacoby G. A., Breckenridge L. Ribosomal ambiguity. Cold Spring Harb Symp Quant Biol. 1966;31:657–664. doi: 10.1101/sqb.1966.031.01.084. [DOI] [PubMed] [Google Scholar]
- HIEROWSKI M. INHIBITION OF PROTEIN SYNTHESIS BY CHLORTETRACYCLINE IN THE E. COLI IN VITRO SYSTEM. Proc Natl Acad Sci U S A. 1965 Mar;53:594–599. doi: 10.1073/pnas.53.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAWETZ E., GUNNISON J. B., SPECK R. S. Studies on antibiotic synergism and antagonism; the interference of aureomycin, chloramphenicol and terramycin with the action of streptomycin. Am J Med Sci. 1951 Oct;222(4):404–412. doi: 10.1097/00000441-195110000-00006. [DOI] [PubMed] [Google Scholar]
- LIGHTBOWN J. W. Metabolic processes underlying streptomycin resistance. G Ital Chemioter. 1957 Jan-Jun;4(1-2):22–32. [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Maxwell I. H. Studies of the binding of tetracycline to ribosomes in vitro. Mol Pharmacol. 1968 Jan;4(1):25–37. [PubMed] [Google Scholar]
- Morris D. W., DeMoss J. A. Role of aminoacyl-transfer ribonucleic acid in the regulation of ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1965 Dec;90(6):1624–1631. doi: 10.1128/jb.90.6.1624-1631.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOTZ P. H., DAVIS B. D. Absence of a chloramphenicol-insensitive phase of streptomycin action. J Bacteriol. 1962 Apr;83:802–805. doi: 10.1128/jb.83.4.802-805.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZYKIN Iu O., CHERNUKH A. M. VYDELENIE I SVO ISTVA SHTAMMA E. COLI, DLIA ROSTA KOTOROGO NEOBKHODIMY STREPTOMITSINOPODOBNYE ANTIBIOTIKI ILI MAKROLIDY. Mikrobiologiia. 1964 Jul-Aug;33:672–678. [PubMed] [Google Scholar]
- SYPHERD P. S., STRAUSS N. THE ROLE OF RNA IN REPRESSION OF ENZYME SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1059–1066. doi: 10.1073/pnas.50.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Davis B. D. Mode of action of novobiocin in Escherichia coli. J Bacteriol. 1967 Jan;93(1):71–79. doi: 10.1128/jb.93.1.71-79.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Suzuka I., Kaji H., Kaji A. Binding of specific sRNA to 30S ribosomal subunits: effect of 50S ribosomal subunits. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1483–1490. doi: 10.1073/pnas.55.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUT R. R., MONRO R. E. THE PUROMYCIN REACTION AND ITS RELATION TO PROTEIN SYNTHESIS. J Mol Biol. 1964 Oct;10:63–72. doi: 10.1016/s0022-2836(64)80028-0. [DOI] [PubMed] [Google Scholar]
- Taubman S. B., Jones N. R., Young F. E., Corcoran J. W. Sensitivity and resistance to erythromycin in Bacillus subtilis 168: the ribosomal binding of erythromycin and chloramphenicol. Biochim Biophys Acta. 1966 Aug 17;123(2):438–440. doi: 10.1016/0005-2787(66)90301-7. [DOI] [PubMed] [Google Scholar]
- Vazquez D. The binding of chloramphenicol by ribosomes from Bacillus megaterium. Biochem Biophys Res Commun. 1964 Apr 22;15(5):464–468. doi: 10.1016/0006-291x(64)90487-5. [DOI] [PubMed] [Google Scholar]
- WHITE J. R., WHITE H. L. STREPTOMYCINOID ANTIBIOTICS: SYNERGISM BY PUROMYCIN. Science. 1964 Nov 6;146(3645):772–774. doi: 10.1126/science.146.3645.772. [DOI] [PubMed] [Google Scholar]
- YAMAKI H., TANAKA N. EFFECTS OF PROTEIN SYNTHESIS INHIBITORS ON THE LETHAL ACTION OF KANAMYCIN AND STREPTOMYCIN. J Antibiot (Tokyo) 1963 Nov;16:222–226. [PubMed] [Google Scholar]