Abstract
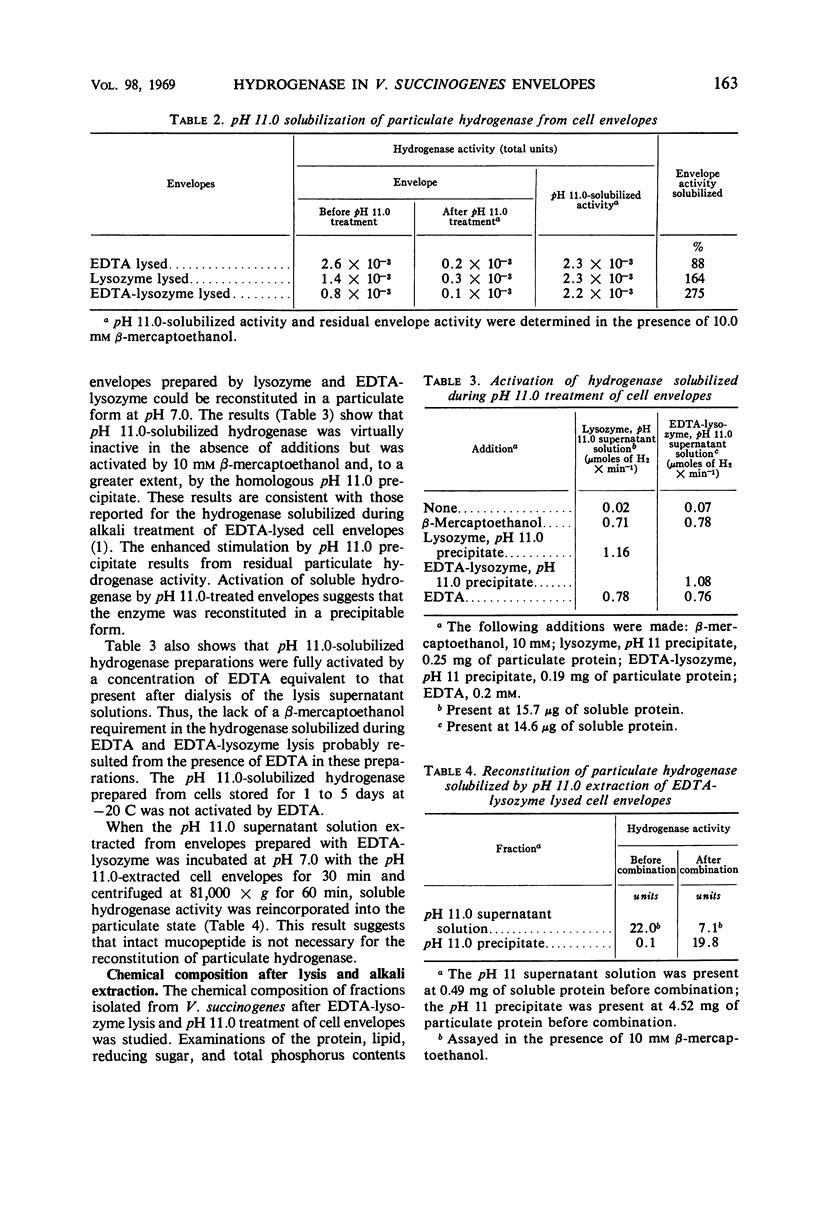
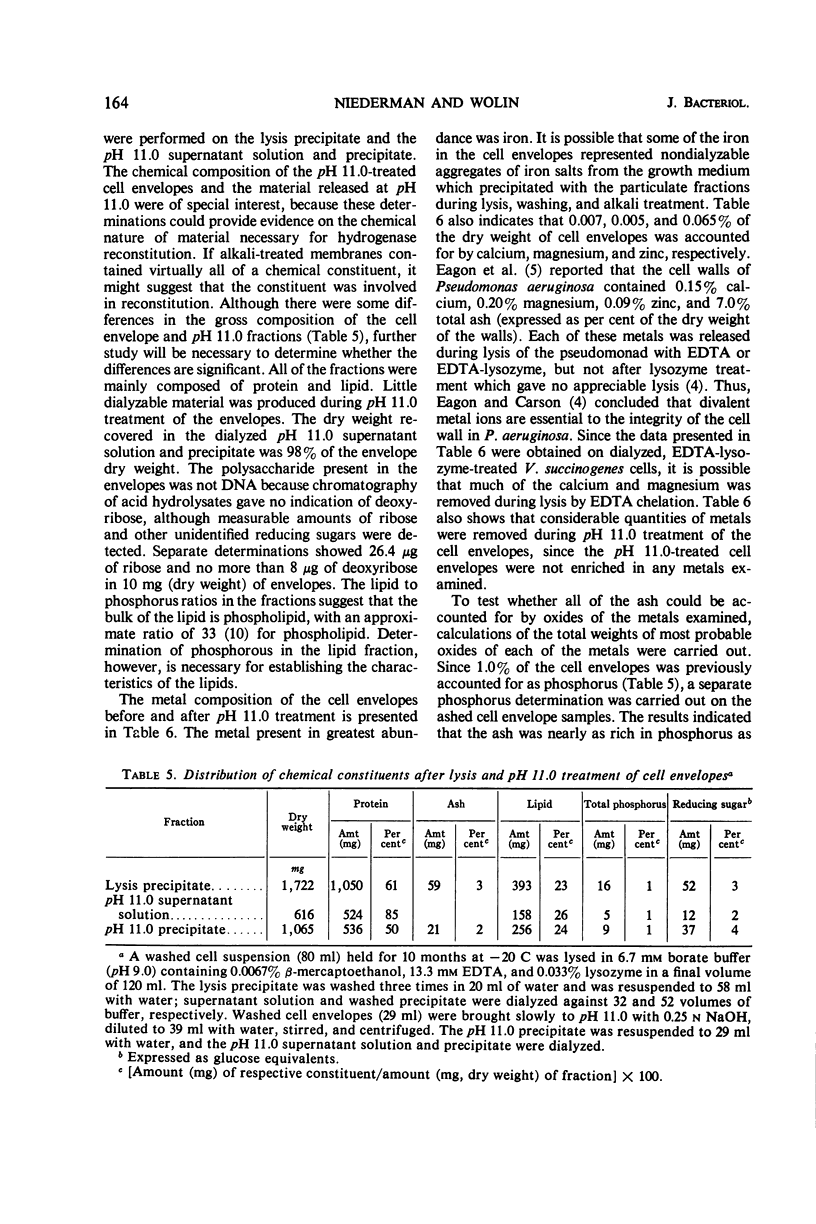
The particulate hydrogenase of Vibrio succinogenes is solubilized during treatment of cell envelopes at pH 11.0. Alkali-solubilized enzyme requires sulfhydryl compounds for activity. At neutral pH, soluble enzyme is reincorporated into alkalitreated cell envelopes and no longer requires an additional activator. In the present study, cell envelopes prepared by lysing cells with ethylenediaminetetraacetic acid plus lysozyme (EDTA-lysozyme) were used to determine the chemical composition of cell envelopes and derived pH 11.0 soluble and insoluble fractions and to investigate some properties of the binding and activation of alkali-solubilized hydrogenase. Lysis with EDTA-lysozyme resulted in the formation of spheroplast ghosts. The derived cell envelopes contained 61% protein, 3% ash, 23% lipid, and 1% phosphorus. The alkali-treated cell envelopes contained 50% protein, 2% ash, 24% lipid, and 1% phosphorus. The ash from cell envelopes and alkali-treated cell envelopes was rich in iron and phosphorus and also contained calcium, copper, magnesium, sodium, and zinc. Virtually all of the weight of the ashed samples was accounted for by the oxides of these metals. Since the reconstitution of particulate hydrogenase was achieved with pH 11.0 supernatant solution and precipitate, intact mucopeptide is not essential for hydrogenase binding. Release of hydrogenase during EDTA-lysozyme lysis was found to depend upon an apparent structural change which occurs in the membranes during extended storage at −20 C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspen A. J., Wolin M. J. Solubilization and reconstitution of a particulate hydrogenase from Vibrio succinogenes. J Biol Chem. 1966 Sep 25;241(18):4152–4156. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Simmons G. P., Carson K. J. Evidence for the presence of ash and fivalent metals in the cell wall of Pseudomonas aeruginosa. Can J Microbiol. 1965 Dec;11(6):1041–1042. doi: 10.1139/m65-144. [DOI] [PubMed] [Google Scholar]
- HUNT A. L., RODGERS A., HUGHES D. E. Sub-cellular particles and the nicotinic acid hydroxylase system in extracts of. Biochim Biophys Acta. 1959 Aug;34:354–372. doi: 10.1016/0006-3002(59)90288-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- ROBRISH S. A., MARR A. G. Location of enzymes in Azotobacteragilis. J Bacteriol. 1962 Jan;83:158–168. doi: 10.1128/jb.83.1.158-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J. Lysis of Vibrio succinogenes by ethylenediamine-tetraacetic acid or lysozyme. J Bacteriol. 1966 May;91(5):1781–1786. doi: 10.1128/jb.91.5.1781-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


