Abstract
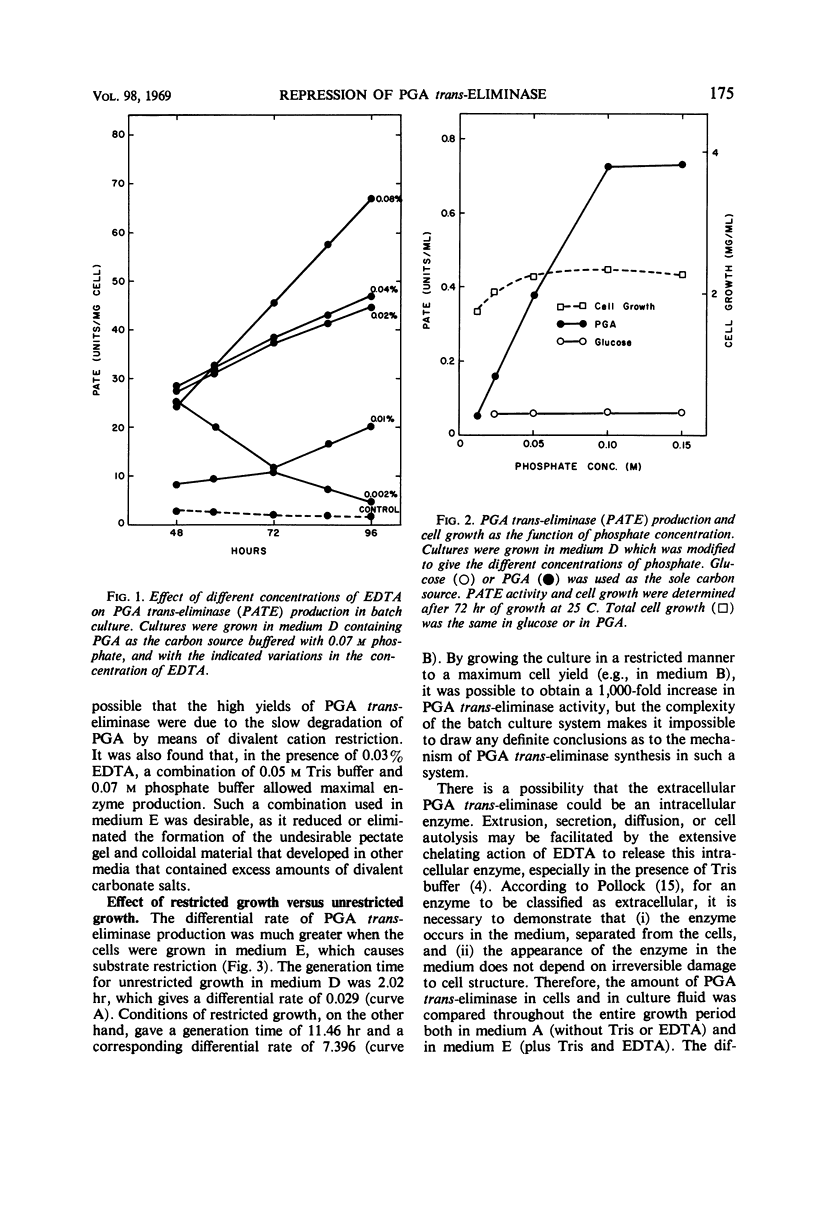
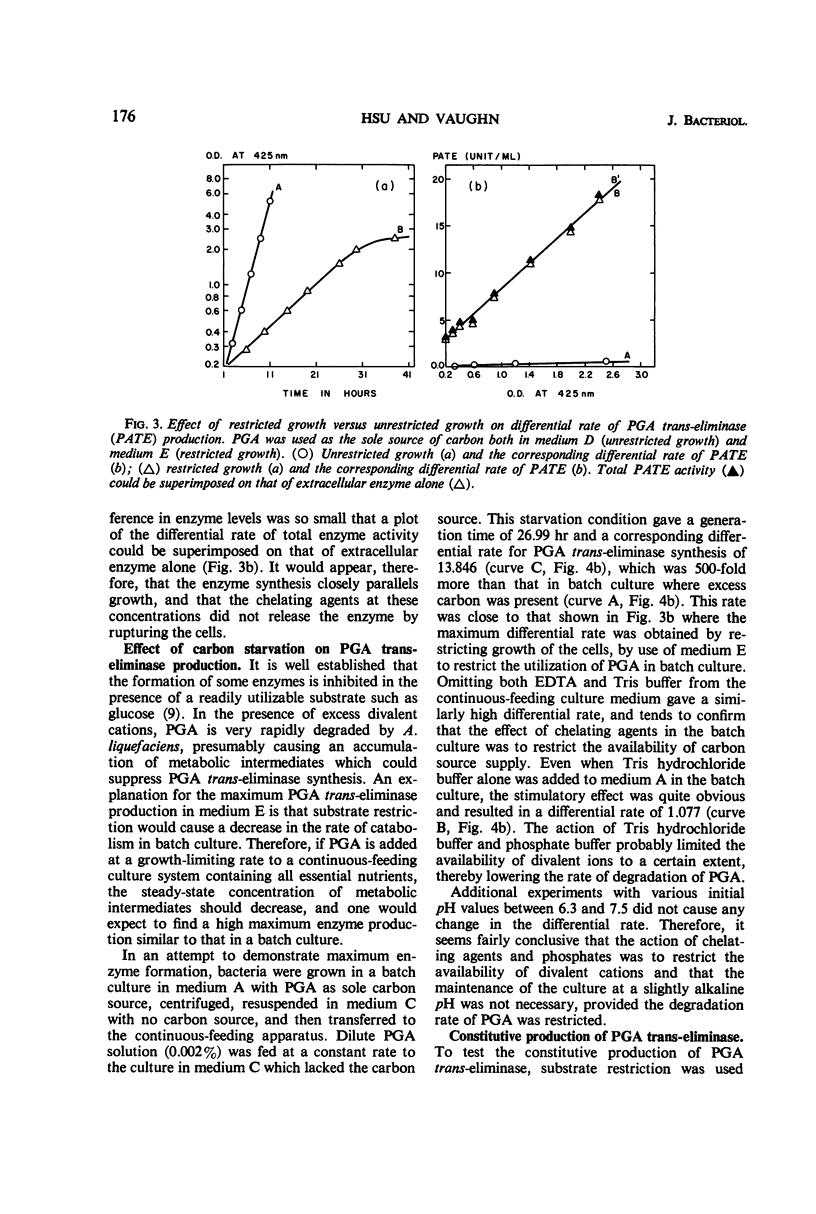
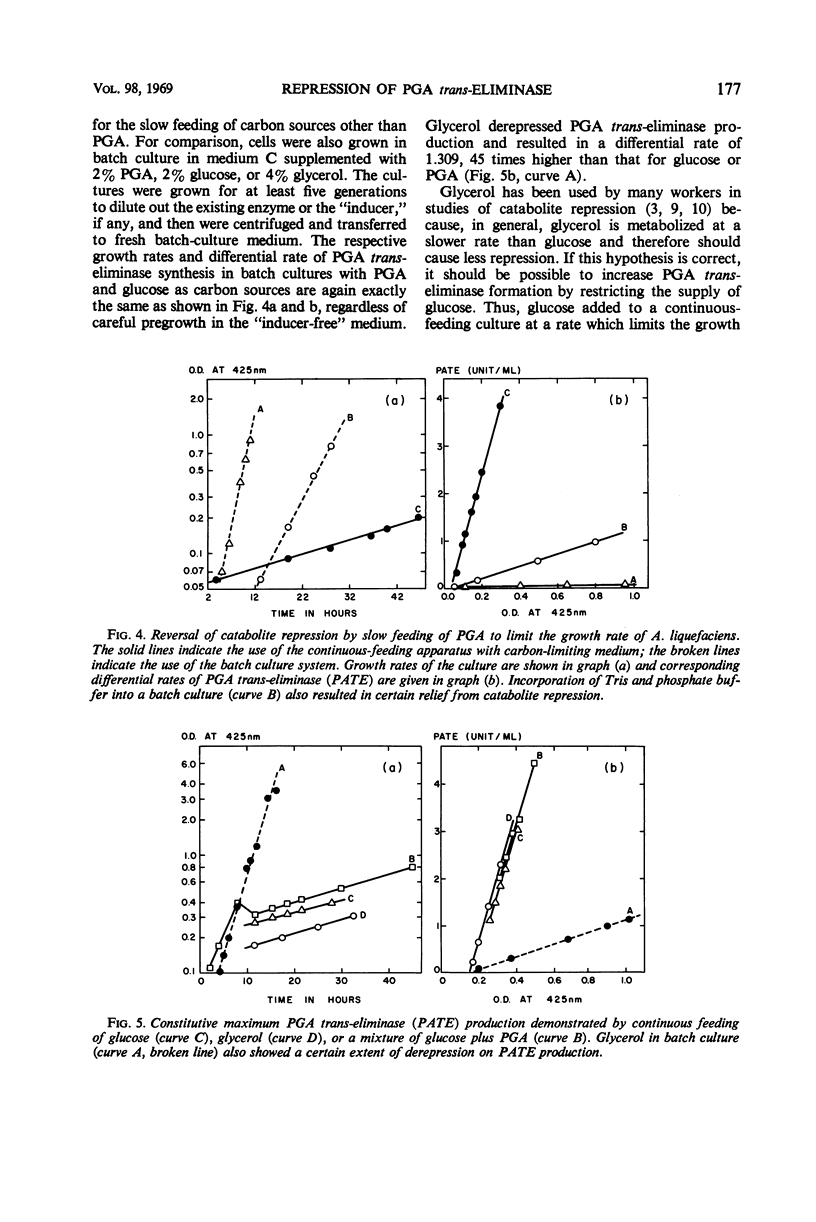
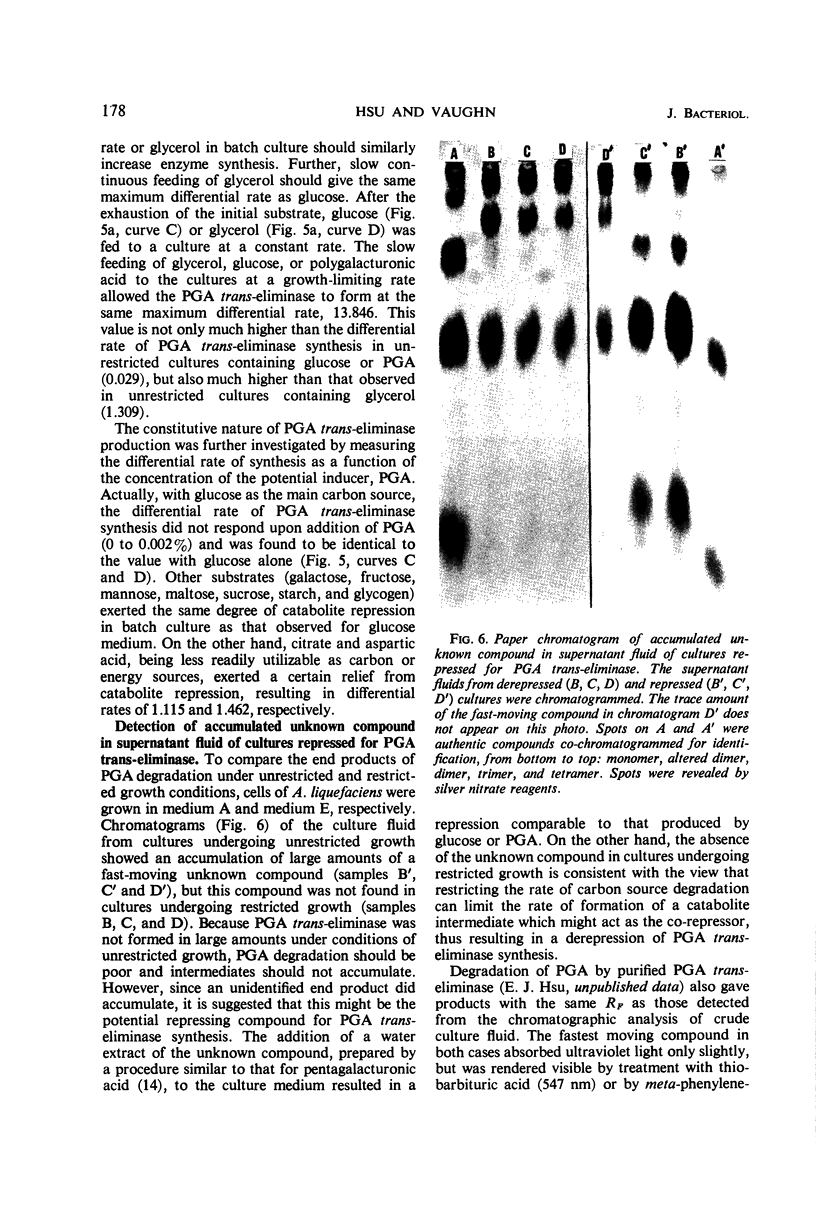
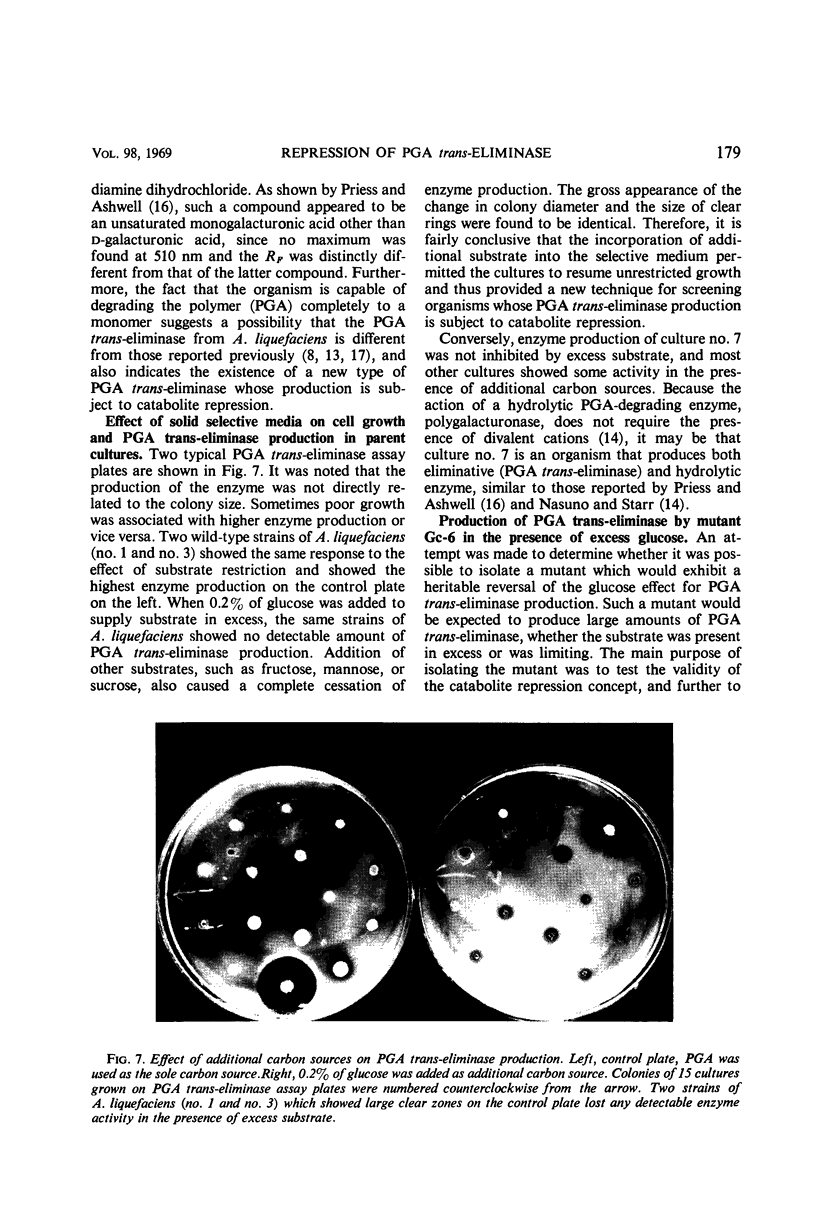
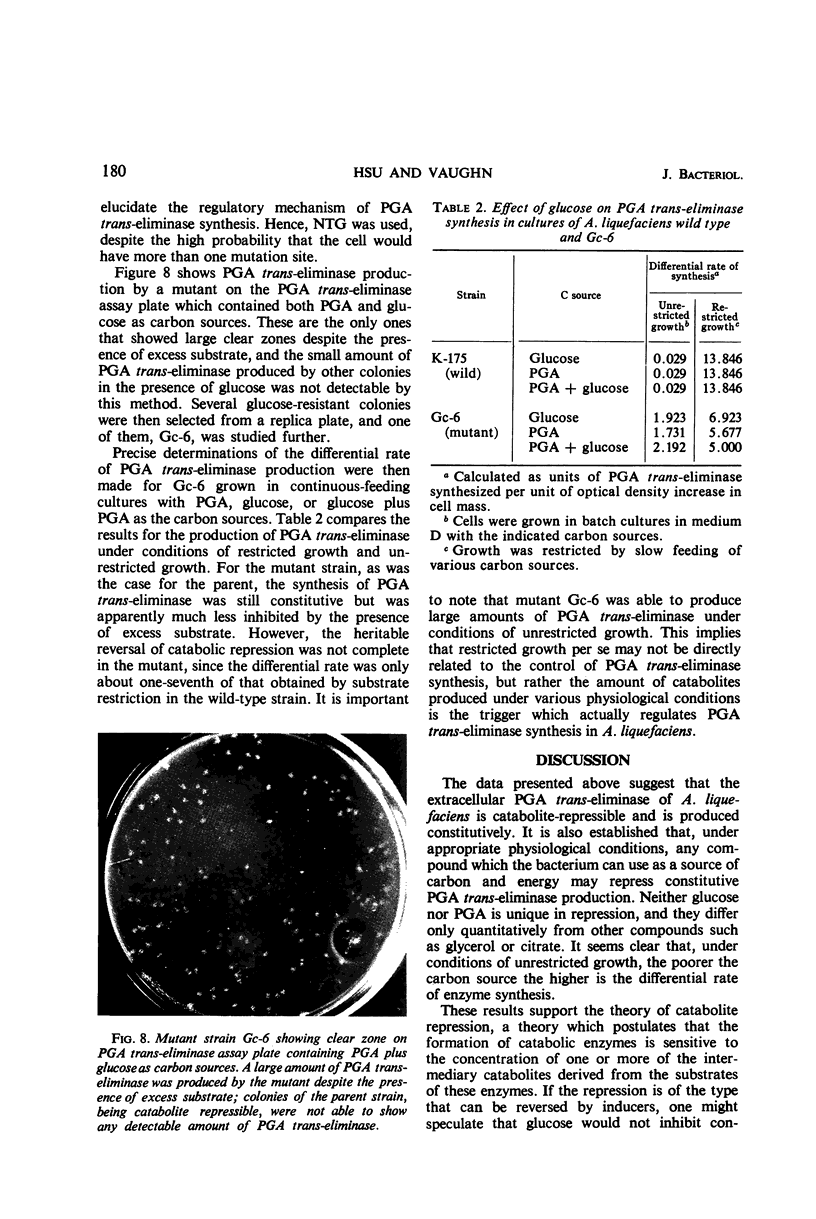
Production of polygalacturonic acid (PGA) trans-eliminase was greatly stimulated under conditions of restricted growth of Aeromonas liquefaciens. This was accomplished either by substrate restriction in a continuous-feeding culture or by restricting divalent cations in a batch culture, with the use of PGA as the sole source of carbon in a chemically defined medium containing inorganic nitrogen. Slow feeding of glucose, glycerol, or PGA to carbon-limited cultures allowed PGA trans-eliminase to be formed at a maximum differential rate 500 times greater than in batch cultures with excess substrate present. The differential rate of enzyme formation obtained by slow feeding of these three substrances or of a mixture of PGA plus glucose was observed to be the same. Therefore, PGA trans-eliminase produced by A. liquefaciens, contrary to the current view, appears to be constitutive. These observations also indicate that production of PGA trans-eliminase is subject to catabolite repression and that limiting the substrate reverses this repression. It was also found that, under conditions of unrestricted growth, any compound which the bacteria can use as a source of carbon and energy repressed constitutive PGA trans-eliminase production. The heritable reversal of catabolite repression of PGA trans-eliminase synthesis was demonstrated by isolation of mutant strain Gc-6 which can readily synthesize the constitutive catabolic enzyme PGA trans-eliminase while growing in the presence of excess substrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERSHEIM P., NEUKOM H. DEUEL H: Splitting of pectin chain molecules in neutral solutions. Arch Biochem Biophys. 1960 Sep;90:46–51. doi: 10.1016/0003-9861(60)90609-3. [DOI] [PubMed] [Google Scholar]
- CLARK D. J., MARR A. G. STUDIES ON THE REPRESSION OF BETA-GALACTOSIDASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Oct 23;92:85–94. doi: 10.1016/0926-6569(64)90272-x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., Wyss O. Chelation effects on Azotobacter cells and cysts. J Bacteriol. 1966 Jan;91(1):120–124. doi: 10.1128/jb.91.1.120-124.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Catabolism of galacturonic and glucuronic acids by Erwinia carotovora. J Biol Chem. 1959 Sep;234:2227–2235. [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. The catabolite repression gene of the lac operon in Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):487–494. doi: 10.1016/s0022-2836(67)80120-7. [DOI] [PubMed] [Google Scholar]
- MACMILLAN J. D., VAUGHN R. H. PURIFICATION AND PROPERTIES OF A POLYGALACTURONIC ACID-TRANS-ELIMINASE PRODUCED BY CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:564–572. doi: 10.1021/bi00892a016. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The characteristics of a polygalacturonase produced by Bacillus polymyxa. Arch Biochem Biophys. 1961 May;93:344–352. doi: 10.1016/0003-9861(61)90277-6. [DOI] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. Catabolite repression and the induction of beta-galactosidase. Biochim Biophys Acta. 1962 Nov 26;61:835–837. doi: 10.1016/0926-6550(62)90070-1. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-L-threo-5-hexoseulose uronic acid. J Biol Chem. 1963 May;238:1571–1583. [PubMed] [Google Scholar]