Abstract
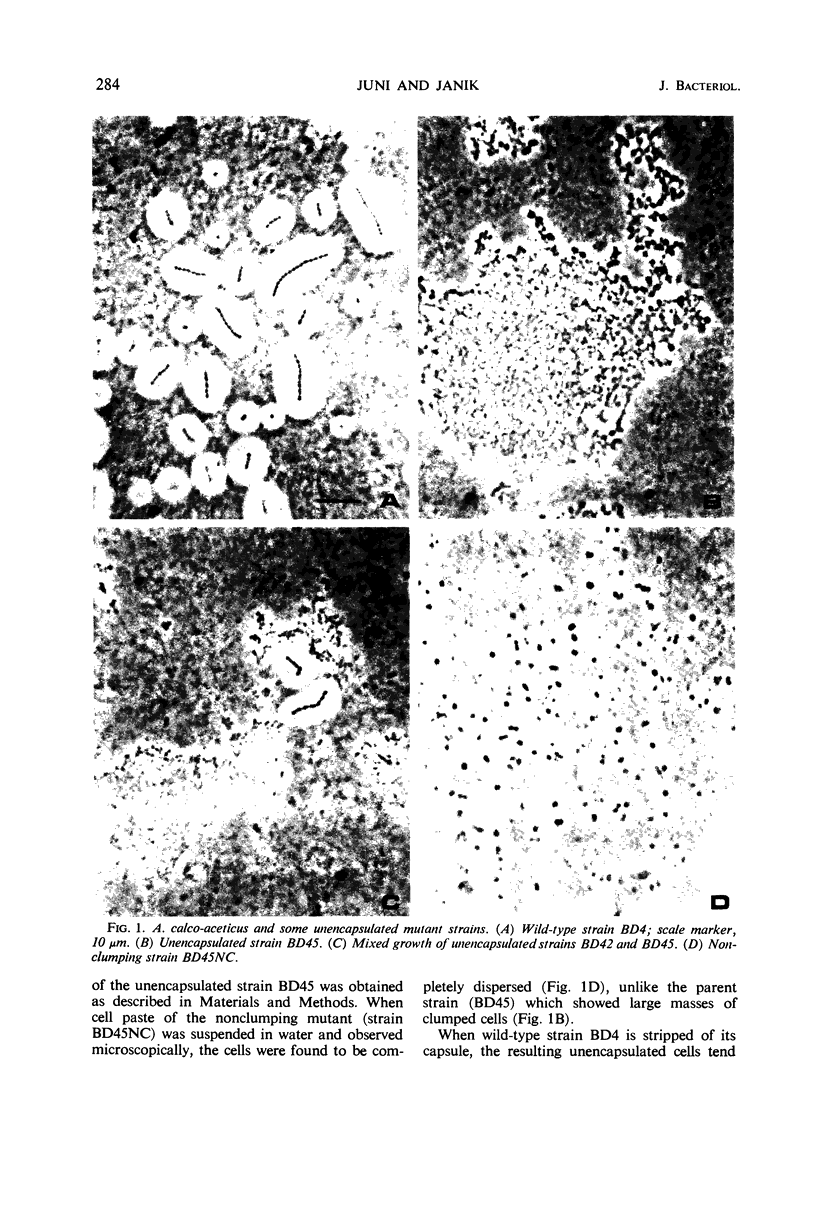
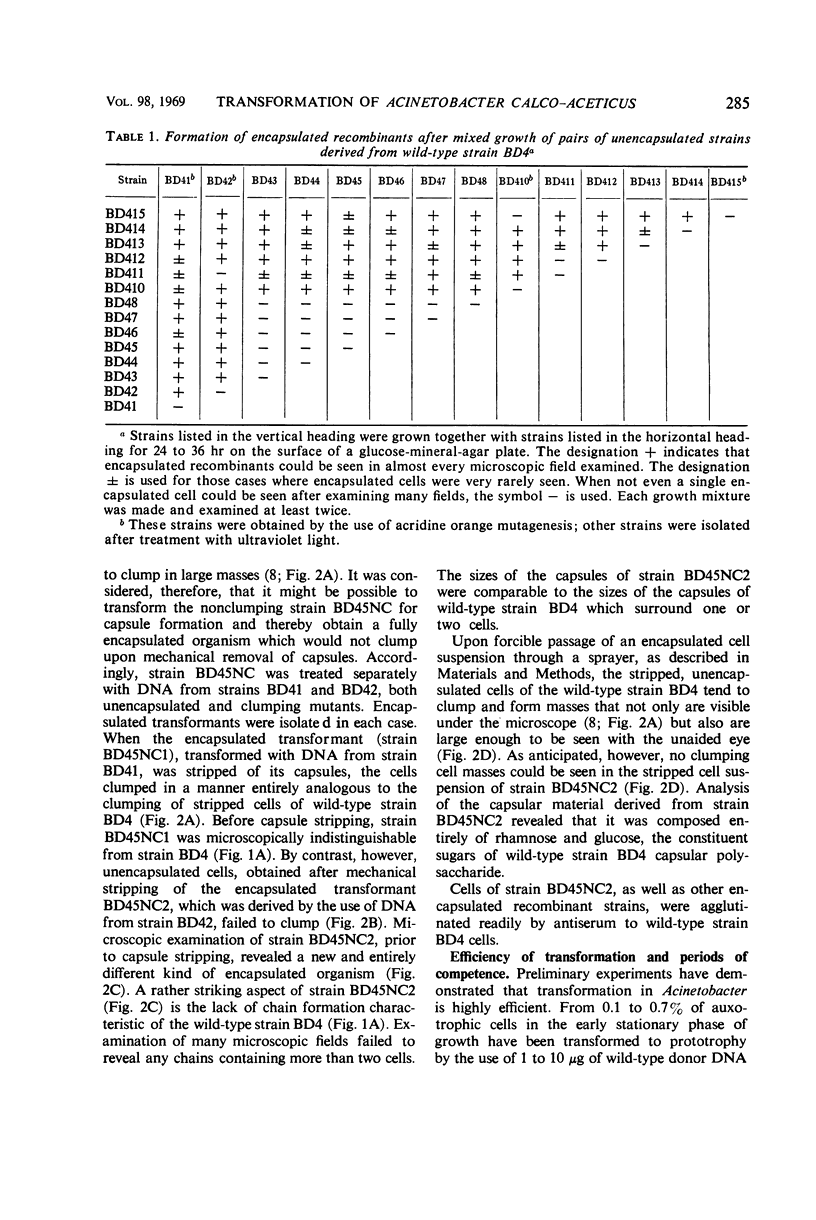
A highly efficient transformation system has been demonstrated in a strain of Acinetobacter calco-aceticus (Bacterium anitratrum). During mixed growth of various stable, unencapsulated, mutant strains, deoxyribonucleic acid (DNA) is liberated and fully encapuslated transformants can be isolated. Purified DNA preparations have been used to transform suitable recipient mutant strains for ability to synthesize capsules, ability to dispense with a growth factor requirement, and resistance to streptomycin. When the wild-type strain is deprived of its capsule, either by mechanical stripping or by mutation, the unencapsulated cells tend to form large clumped masses. A nonclumping mutant of an unencapsulated strain has been isolated. When ability to synthesize capsules is transformed into this nonclumping strain, the resultant cells no longer form chains, unlike the wild-type encapsulated strain. It appears likely that the occurrence of transformation during growth of mixed cultures, with glucose or gluconate as the carbon source, may be the result of osmotic rupture resulting from the inability of unencapsulated strains to oxidize triose phosphates as fast as they are formed. The finding of transformation in Acinetobacter may provide an additional useful organism for the study of this mode of genetic transfer since this strain grows well in a simple mineral medium containing a single oxidizable source of carbon. Furthermore, no special supplementary factors seem to be required for transformation to take place.
Full text
PDF


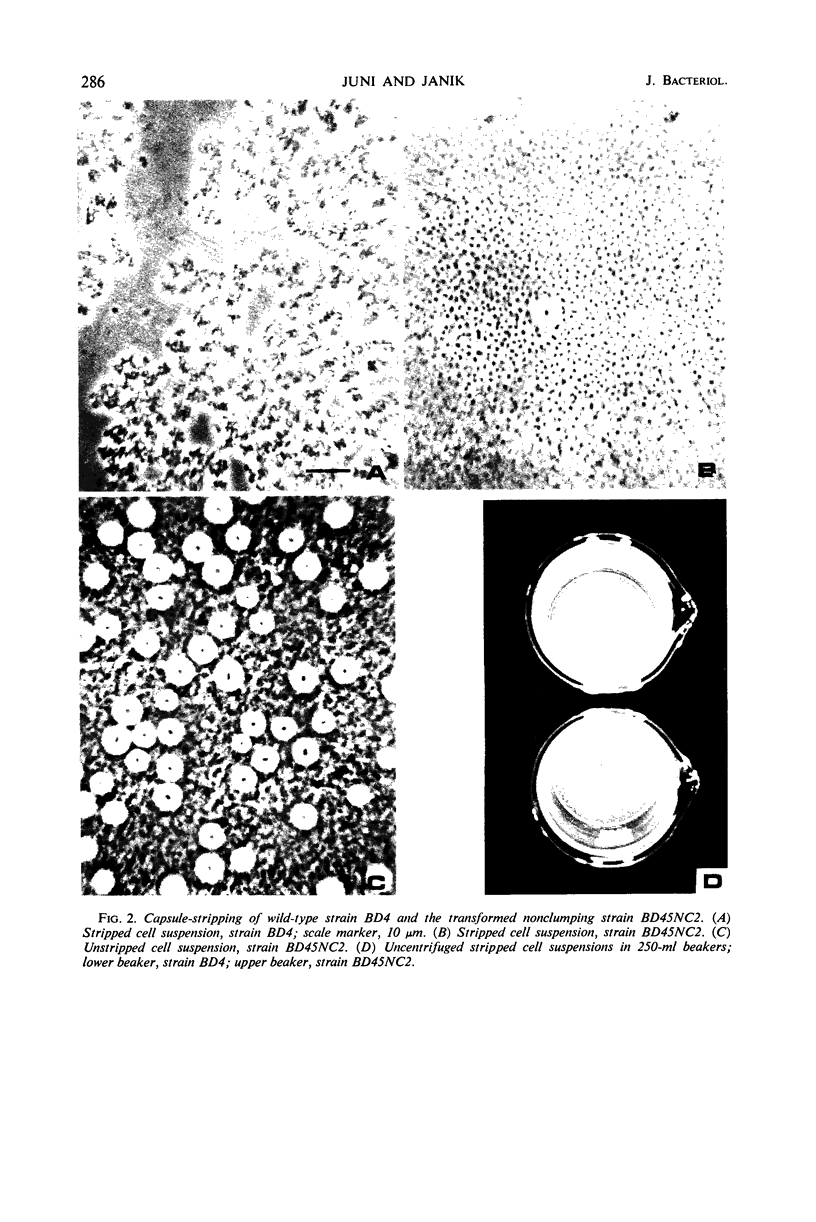


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P., SMITH E. E., MILLS G. T. Simultaneous production of two capsular polysaccharides by pneumococcus. II. The genetic and biochemical bases of binary capsulation. J Exp Med. 1959 Oct 1;110:585–602. doi: 10.1084/jem.110.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEVRE K. STUDIES ON TRANSFORMATION IN MORAXELLA AND ORGANISMS ASSUMED TO BE RELATED TO MORAXELLA. 1. A METHOD FOR QUANTITATIVE TRANSFORMATION IN MORAXELLA AND NEISSERIA, WITH STREPLOMYCIN RESISTANCE AS THE GENETIC MARKER. Acta Pathol Microbiol Scand. 1964;61:457–473. doi: 10.1111/apm.1964.61.3.457. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951 Oct;63(4):673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- GWINN D. D., THORNE C. B. TRANSFORMATION OF BACILLUS LICHENIFORMIS. J Bacteriol. 1964 Mar;87:519–526. doi: 10.1128/jb.87.3.519-526.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. PATHWAYS FOR BIOSYNTHESIS OF A BACTERIAL CAPSULAR POLYSACCHARIDE. IV. CAPSULE RESYNTHESIS BY DECAPSULATED RESTING-CELL SUSPENSIONS. J Bacteriol. 1964 Feb;87:461–467. doi: 10.1002/path.1700870234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Carbohydrate metabolism and terminal oxidation mechanisms of a capsuleproducing coccus. J Bacteriol. 1961 May;81:694–703. doi: 10.1128/jb.81.5.694-703.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961 May;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]