Abstract
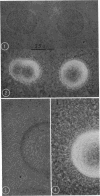
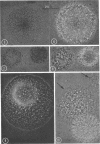
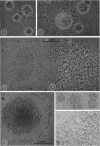
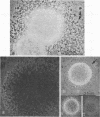
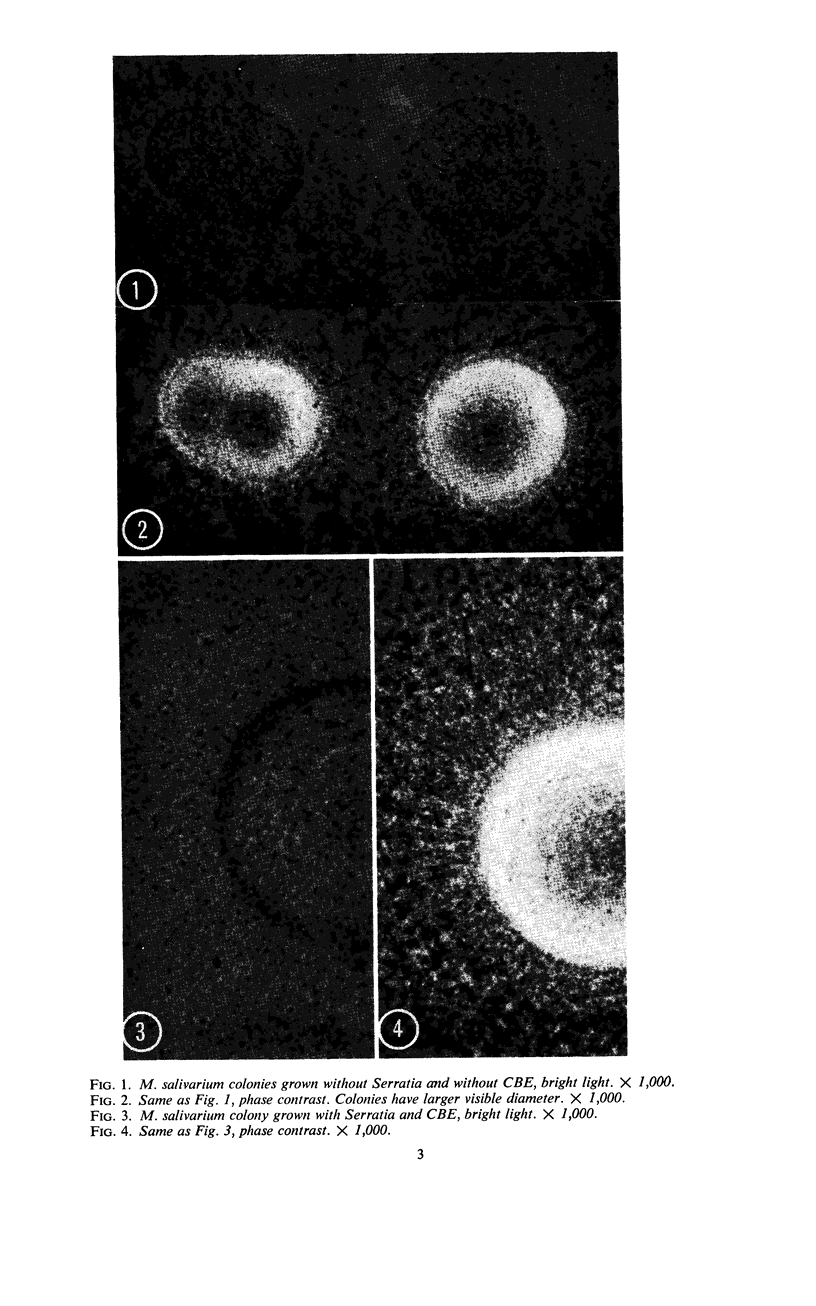
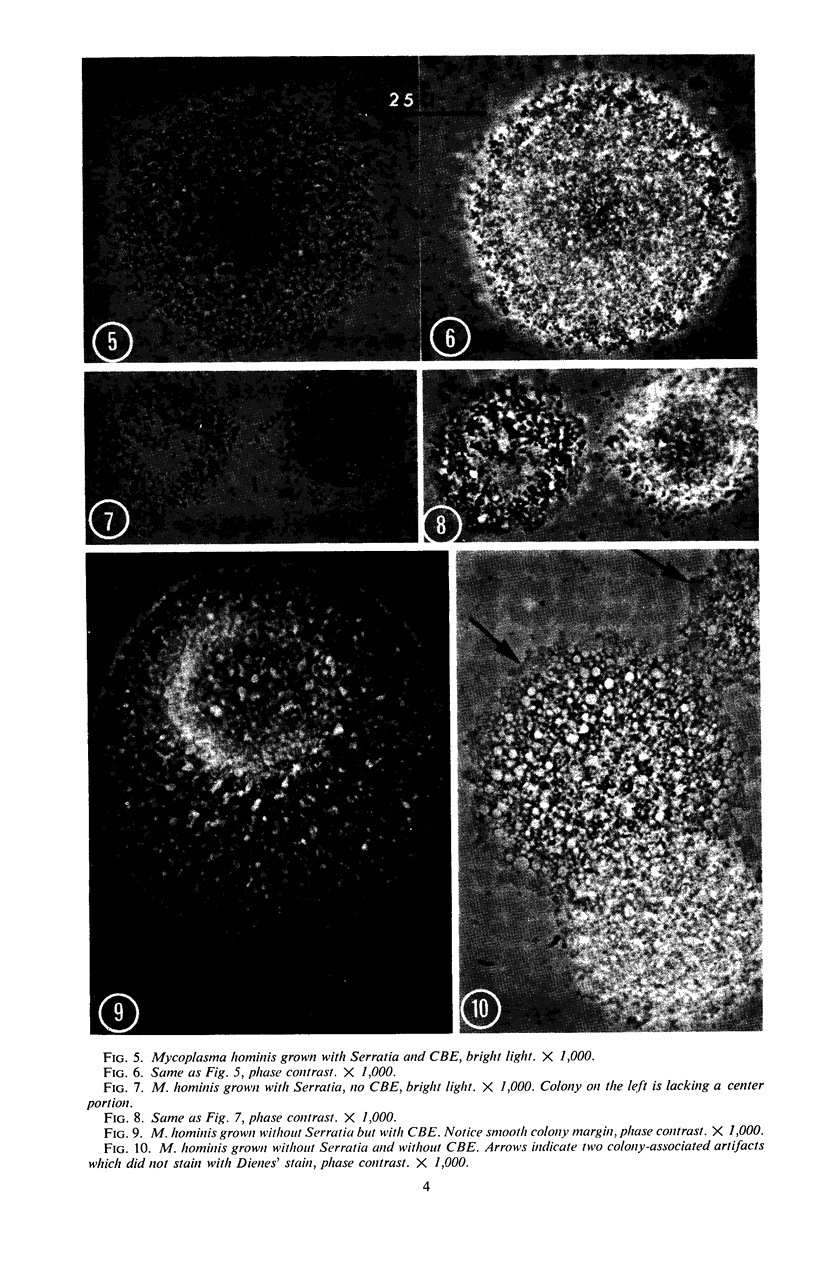
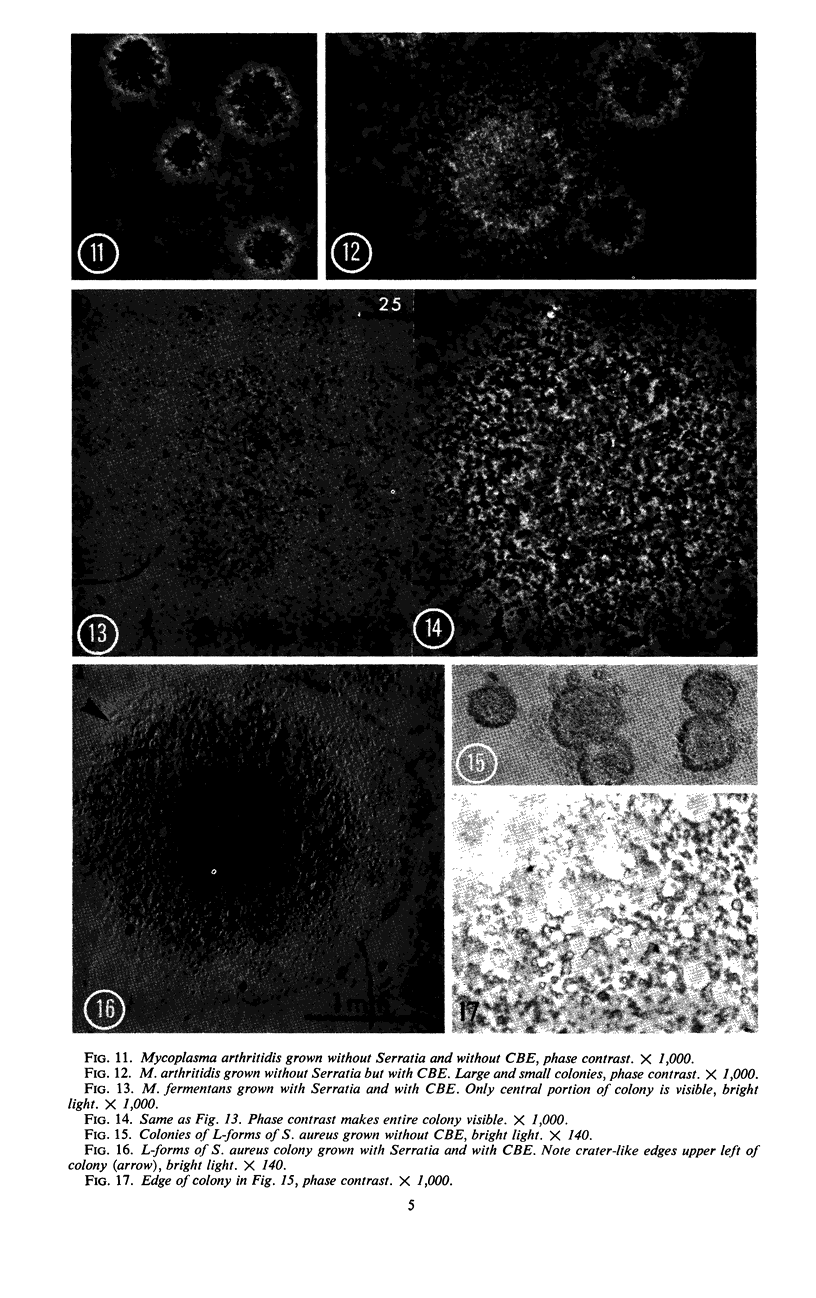
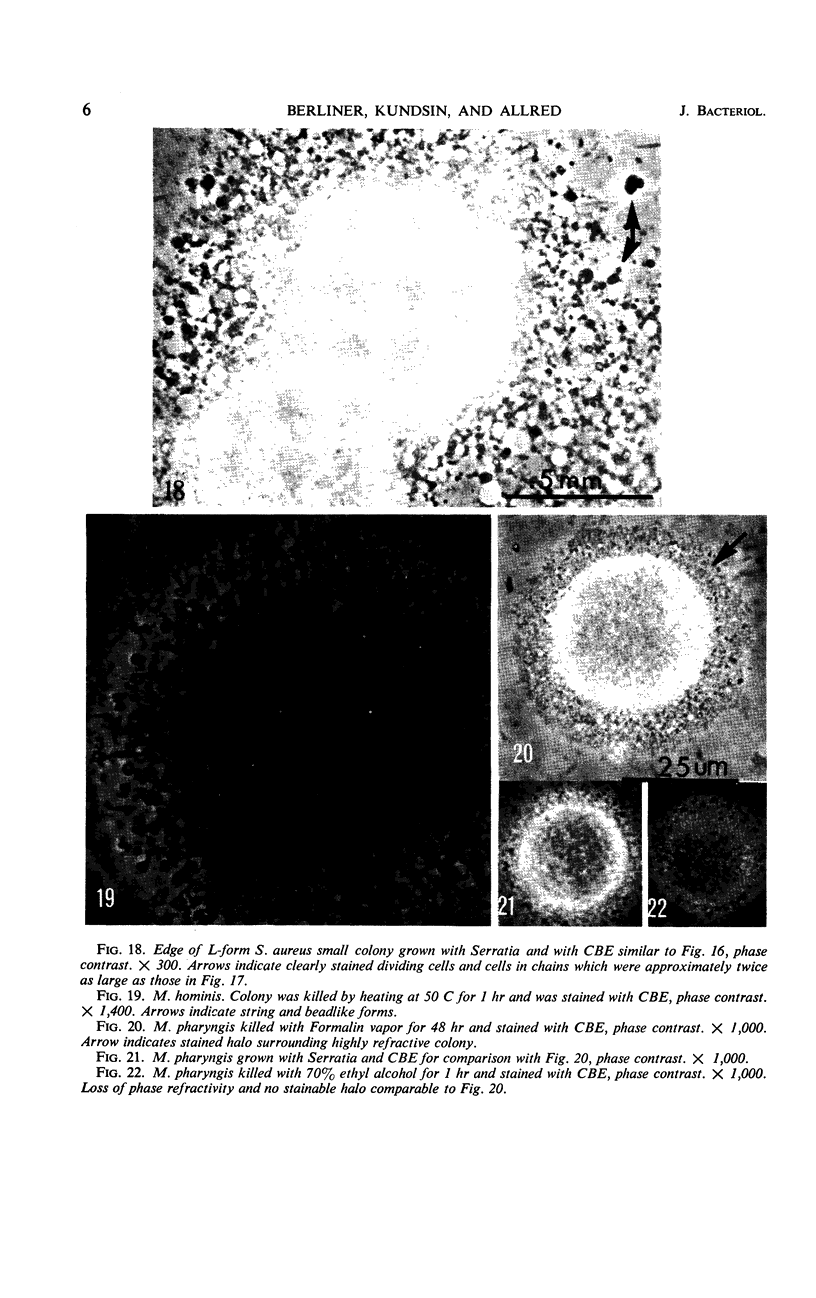
Vital staining of Mycoplasma colonies was attempted because other dye visualization techniques kill the organisms and preclude reisolation for further studies. The lipophilic amphoteric dye Chlorazol Black E (CBE) was the most successful of 14 vital dyes tested on Mycoplasma hominis, M. pharyngis, M. fermentans, M. arthritidis, M. salivarium, M. pneumoniae, and L-forms of Staphylococcus aureus when used in 1:1,000 (w/v) saline dilution as the sterile suspension medium for inoculation of Hayflick's medium under both aerobic and microaerophilic (Fortner method) conditions. Colonies of all species stain homogeneously in the periphery and center portion, the latter being more refractive under positive phase contrast. All stained colonies were successfully subcultured. The most striking and promising result of the use of CBE as a tool for physiological study of Mycoplasma was a very significant increase in diameter of all colonies except those of M. pneumoniae grown with CBE: 1.5 × for M. hominis and 5 × for L-form S. aureus. This size increase in M. hominis is proportional to the concentration down to a 1:50,000 dilution only under microaerophilic conditions. Whether this increase in colony size is due to an increased number of cells, to larger cells, or to the adsorption of CBE on the lipid membrane is unknown at present.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner M. D., Reca M. E. Vital staining of Histoplasma capsulatum with Janus Green B. Sabouraudia. 1966 Jun;5(1):26–29. [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes L. Permanent stained agar preparation of Mycoplasma and of L forms of bacteria. J Bacteriol. 1967 Feb;93(2):689–692. doi: 10.1128/jb.93.2.689-692.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., CHANOCK R. M. MYCOPLASMA SPECIES OF MAN. Bacteriol Rev. 1965 Jun;29:185–221. doi: 10.1128/br.29.2.185-221.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan G. Y. The results of some comparative experimental investigations of L-forms of bacteria and mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):734–748. doi: 10.1111/j.1749-6632.1967.tb27720.x. [DOI] [PubMed] [Google Scholar]
- Knaysi G. A Microscopic Method of Distinguishing Dead from Living Bacterial Cells. J Bacteriol. 1935 Aug;30(2):193–206. doi: 10.1128/jb.30.2.193-206.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADOFF S. Isolation and identification of PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:383–392. doi: 10.1111/j.1749-6632.1960.tb42702.x. [DOI] [PubMed] [Google Scholar]
- Razin S. The cell membrane of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):115–129. doi: 10.1111/j.1749-6632.1967.tb27651.x. [DOI] [PubMed] [Google Scholar]