Abstract
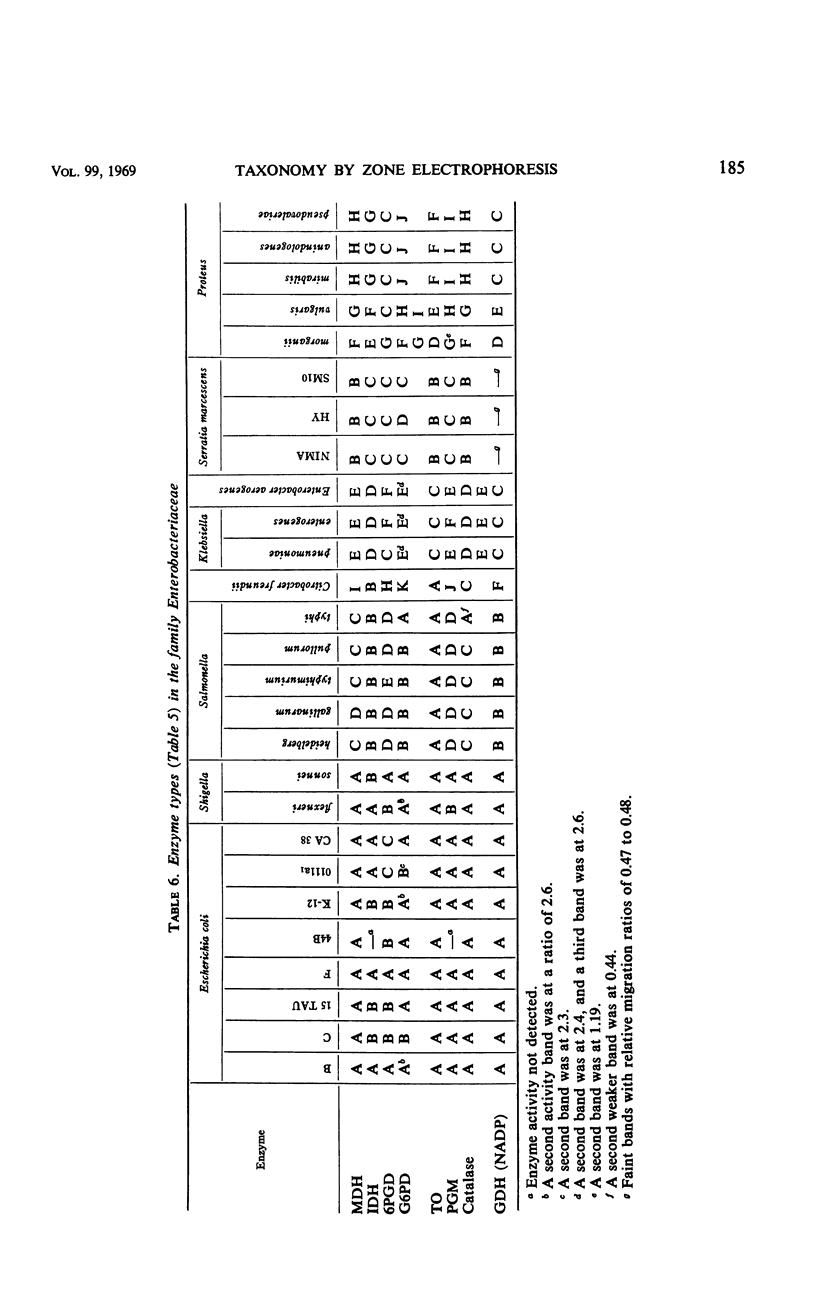
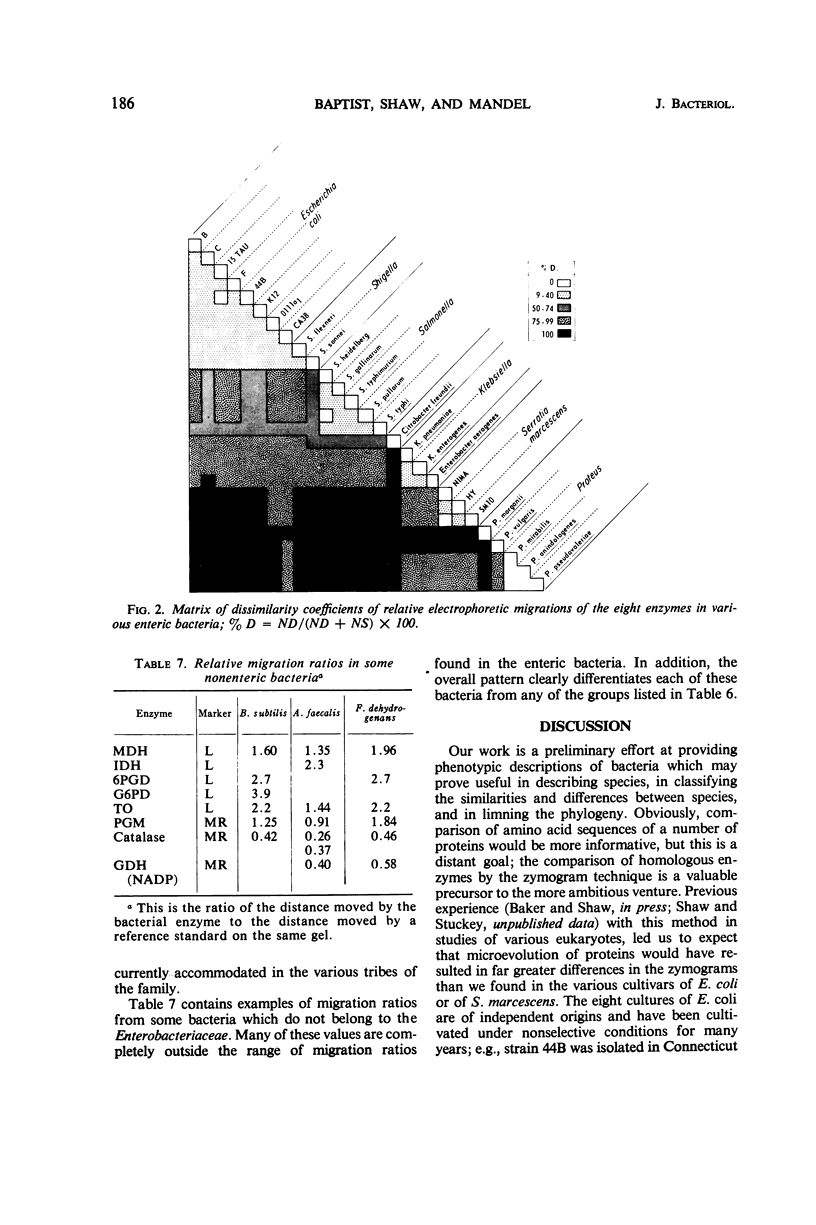
The electrophoretic mobilities in starch gels have been determined for eight enzymes in extracts of representative cultures of members of the family Enterobacteriaceae. These values were compared with each other and with those obtained from certain bacteria not in this family. The migrations of the eight enzymes were virtually identical for each of eight strains of Escherichia coli and for two species of Shigella. A number of these enzymes appeared to be identical in other organisms believed to be closely related to E. coli (Salmonella), and the number of differences increased in organisms which appeared to have lesser degrees of relatedness by other criteria (deoxyribonucleic acid base compositions, overall similarity).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Asato R. N., Mower H. F. Multiple forms of bacterial hydrogenases. J Bacteriol. 1966 Oct;92(4):828–838. doi: 10.1128/jb.92.4.828-838.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison W. S. Structure and evolution of triose phosphate and lactate dehydrogenases. Ann N Y Acad Sci. 1968 Jun 14;151(1):180–189. doi: 10.1111/j.1749-6632.1968.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Baron L. S., Gemski P., Johnson E. M., Wohlhieter J. A. Intergeneric bacterial matings. Bacteriol Rev. 1968 Dec;32(4 Pt 1):362–369. [PMC free article] [PubMed] [Google Scholar]
- Bowman J. E., Brubaker R. R., Frischer H., Carson P. E. Characterization of enterobacteria by starch-gel electrophoresis of glucose-6-phosphate dehydrogenase and phosphogluconate dehydrogenase. J Bacteriol. 1967 Sep;94(3):544–551. doi: 10.1128/jb.94.3.544-551.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R. R., Adeyemo V. I., Kirtland H. H. Esterases and DNA base composition analysis of Vibrio cholerae and related vibrios. J Appl Bacteriol. 1968 Sep;31(3):323–335. doi: 10.1111/j.1365-2672.1968.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Falkow S., Rownd R., Baron L. S. GENETIC HOMOLOGY BETWEEN ESCHERICHIA COLI K-12 AND SALMONELLA. J Bacteriol. 1962 Dec;84(6):1303–1312. doi: 10.1128/jb.84.6.1303-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER R. L., MARKERT C. L. Histochemical demonstration of enzymes separated by zone electrophoresis in starch gels. Science. 1957 Jun 28;125(3261):1294–1295. doi: 10.1126/science.125.3261.1294-a. [DOI] [PubMed] [Google Scholar]
- Kitto G. B., Wassarman P. M., Kaplan N. O. Enzymatically active conformers of mitochondrial malate dehydrogenase. Proc Natl Acad Sci U S A. 1966 Aug;56(2):578–585. doi: 10.1073/pnas.56.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg R. E., Lockhart W. R. Classification of enterobacteria based on overall similarity. J Bacteriol. 1966 Nov;92(5):1275–1280. doi: 10.1128/jb.92.5.1275-1280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B. M. A comparison by the use of gel electrophoresis of soluble protein components and esterase enzymes of some group D Streptococci. J Gen Microbiol. 1965 Sep;40(3):413–419. doi: 10.1099/00221287-40-3-413. [DOI] [PubMed] [Google Scholar]
- Mandel M. Deoxyribonucleic acid base composition in the genus Pseudomonas. J Gen Microbiol. 1966 May;43(2):273–292. doi: 10.1099/00221287-43-2-273. [DOI] [PubMed] [Google Scholar]
- Morichi T., Sharpe M. E., Reiter B. Esterases and other soluble proteins of some lactic acid bacteria. J Gen Microbiol. 1968 Oct;53(3):405–414. doi: 10.1099/00221287-53-3-405. [DOI] [PubMed] [Google Scholar]
- Razin S. Mycoplasma taxonomy studiedy electrophoresis of cell proteins. J Bacteriol. 1968 Sep;96(3):687–694. doi: 10.1128/jb.96.3.687-694.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Rottem S. Identification of Mycoplasma and other microorganisms by polyacrylamide-gel electrophoresis of cell proteins. J Bacteriol. 1967 Dec;94(6):1807–1810. doi: 10.1128/jb.94.6.1807-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER H., FALKOW S. CHARACTERIZATION OF AN HFR STRAIN OF SHIGELLA FLEXNERI. J Bacteriol. 1964 Sep;88:682–689. doi: 10.1128/jb.88.3.682-689.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Intracytoplasmic membranes in Escherichia coli. J Bacteriol. 1966 Sep;92(3):780–783. doi: 10.1128/jb.92.3.780-783.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. R. Electrophoretic variation in enzymes. Science. 1965 Aug 27;149(3687):936–943. doi: 10.1126/science.149.3687.936. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. A. LOCATION OF GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE IN STARCH-GELS. Nature. 1964 Sep 5;203:1070–1071. doi: 10.1038/2031070a0. [DOI] [PubMed] [Google Scholar]
- Williams R. A., Bowden E. The starch-gel electrophoresis of glucose-6-phosphate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase of Streptococcus faecalis, S. faecium and S. durans. J Gen Microbiol. 1968 Feb;50(2):329–336. doi: 10.1099/00221287-50-2-329. [DOI] [PubMed] [Google Scholar]