Abstract
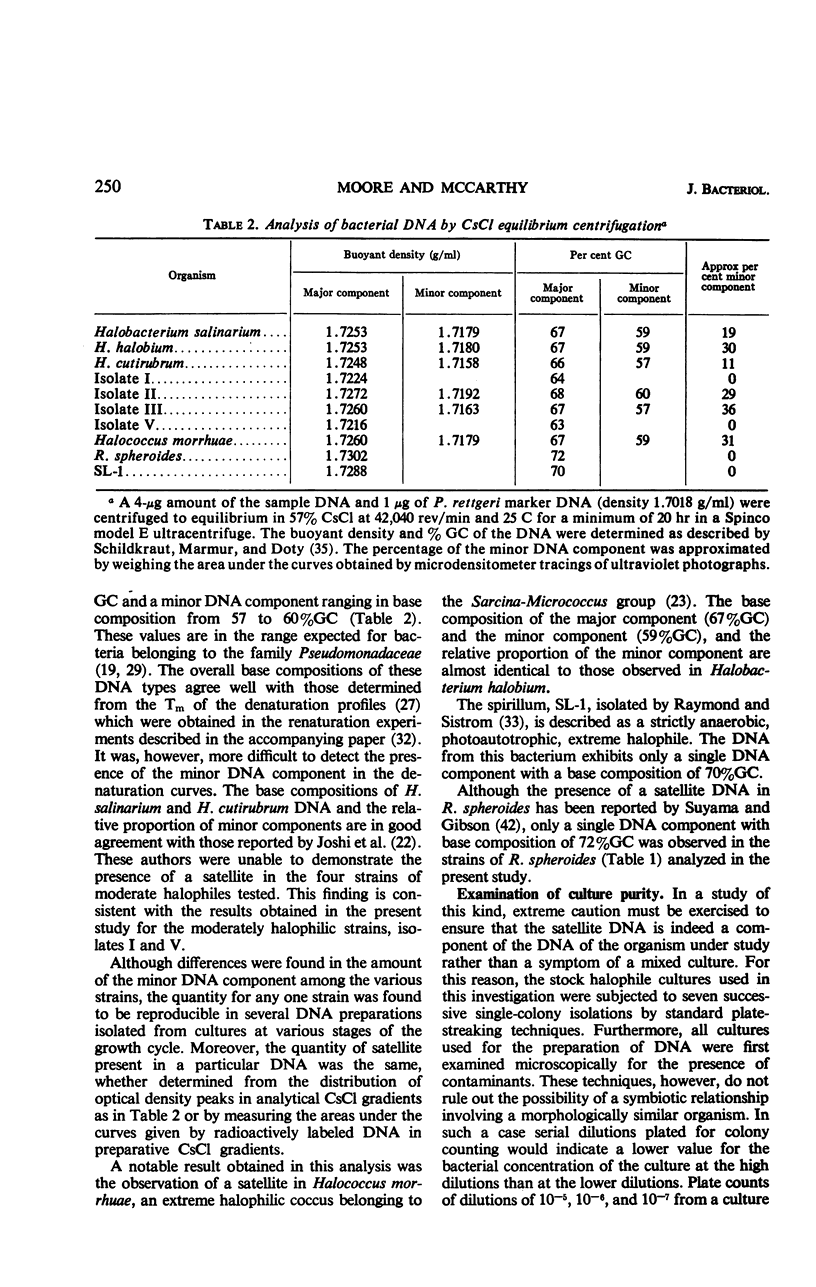
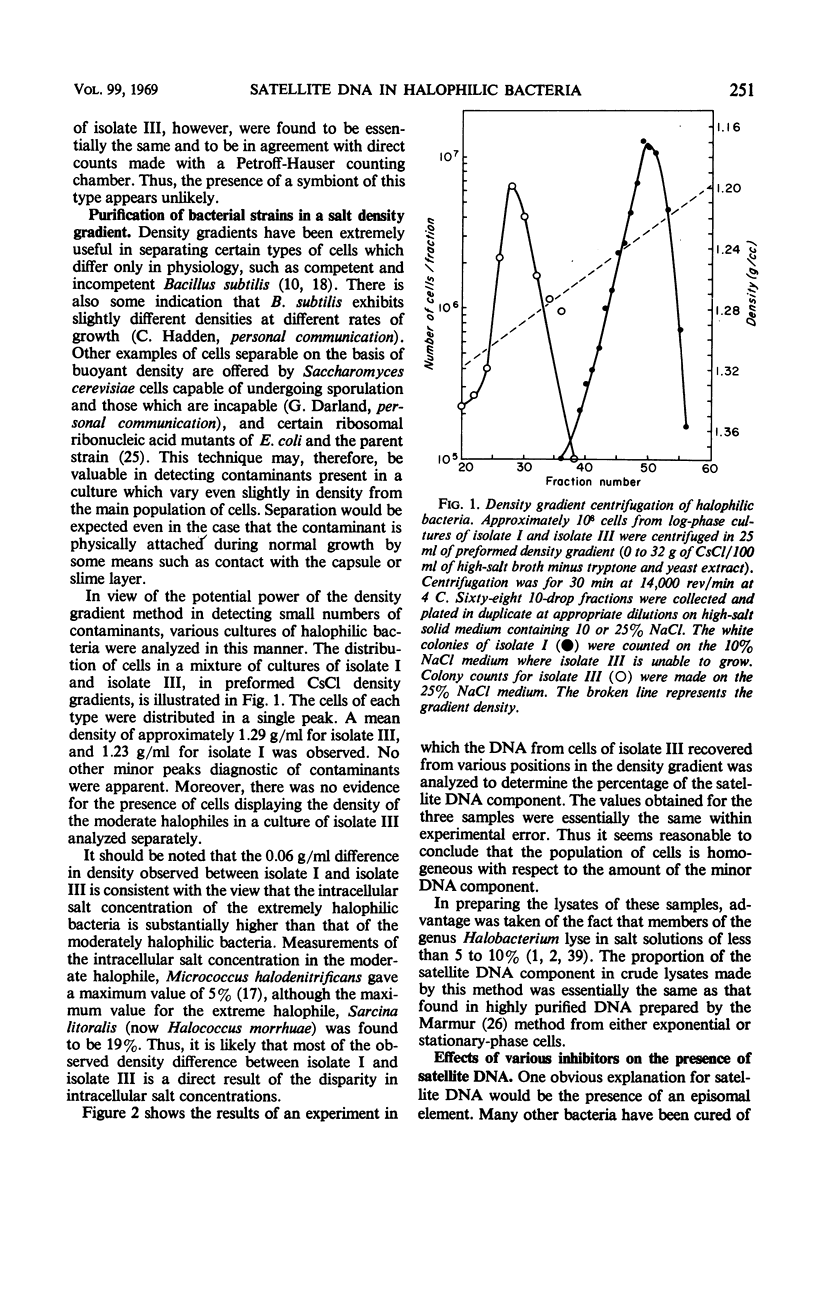
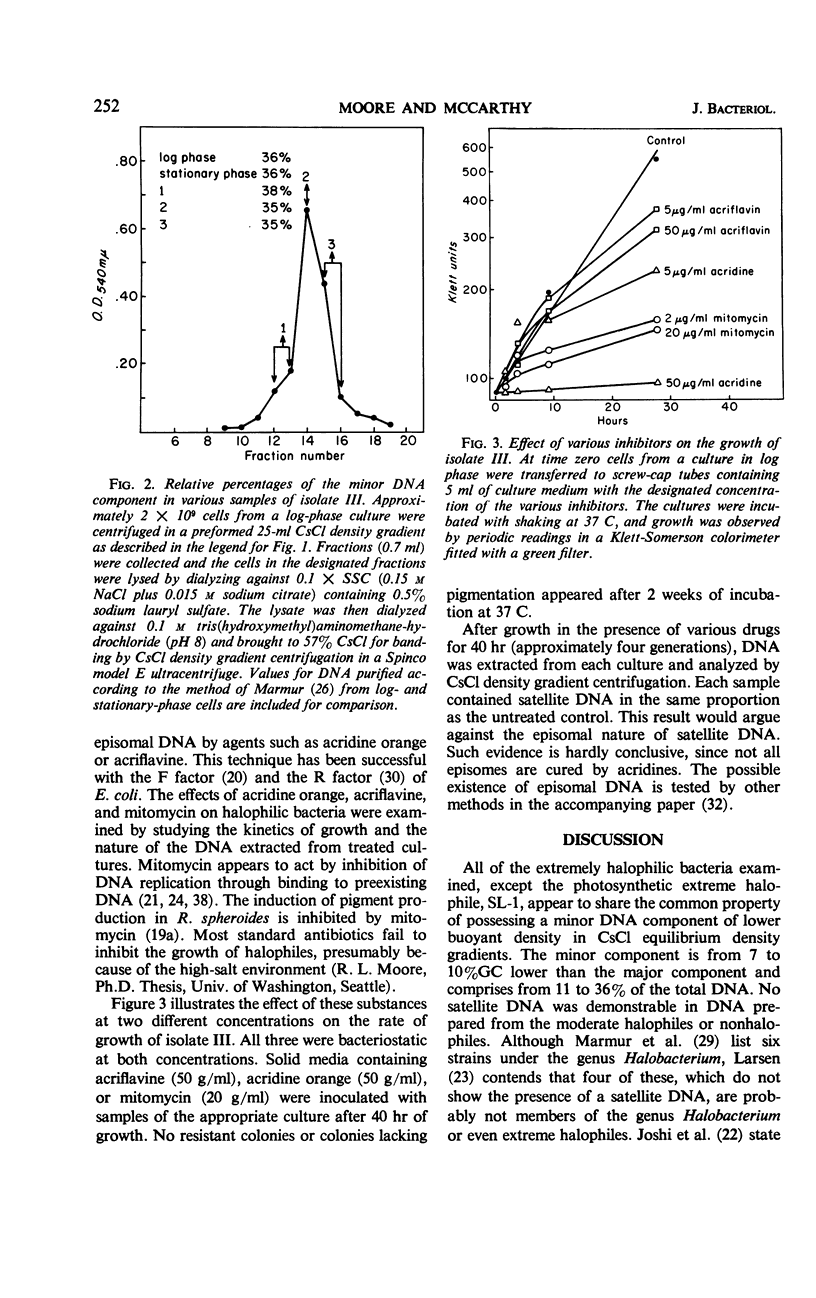
Bacteria classified as extreme halophiles, in the genera Halobacterium and Halococcus, contain deoxyribonucleic acid (DNA) which displays two components in a CsCl equilibrium density gradient. The base composition of the major DNA component ranges from 66 to 68% guanine plus cytosine (GC), whereas that of the satellite DNA comprising some 11 to 36% of the total, is between 57 and 60% GC. Purification of the bacterial cells in a CsCl density gradient and other more conventional strain purification procedures both indicated that the presence of the satellite DNA component is not a result of mixed cultures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., GIBBONS N. E. The effect of chlorides of monovalent cations, urea, detergents, and heat on morphology and the turbidity of suspensions of red halophilic bacteria. Can J Microbiol. 1961 Oct;7:741–750. doi: 10.1139/m61-088. [DOI] [PubMed] [Google Scholar]
- ABRAM D., GIBBONS N. E. Turbidity of suspensions and morphology of red halophilic bacteria as influenced by sodium chloride concentration. Can J Microbiol. 1960 Oct;6:535–543. doi: 10.1139/m60-062. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M., GIBBONS N. E. Effects of sodium and potassium chloride on certain enzymes of Micrococcus halodenitrificans and Pseudomonas salinaria. Can J Microbiol. 1956 Oct;2(6):599–606. doi: 10.1139/m56-072. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M., GIBBONS N. E. The cysteine desulphydrase of Pseudomonas salinaria. Can J Microbiol. 1957 Apr;3(3):461–465. doi: 10.1139/m57-049. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M., GIBBONS N. E. The glycerol dehydrogenases of Pseudomonas salinaria, Vibrio costicolus, and Escherichia coli in relation to bacterial halophilism. Can J Biochem Physiol. 1954 May;32(3):206–217. [PubMed] [Google Scholar]
- BAYLEY S. T., KUSHNER D. J. THE RIBOSOMES OF THE EXTREMELY HALOPHILIC BACTERIUM, HALOBACTERIUM CUTIRUBRUM. J Mol Biol. 1964 Sep;9:654–669. doi: 10.1016/s0022-2836(64)80173-x. [DOI] [PubMed] [Google Scholar]
- BROWN H. J., GIBBONS N. E. The effect of magnesium, potassium, and iron on the growth and morphology of red halophilic bacteria. Can J Microbiol. 1955 Aug;1(7):486–494. doi: 10.1139/m55-062. [DOI] [PubMed] [Google Scholar]
- Bayley S. T., Griffiths E. A cell-free amino acid incorporating system from an extremely halophilic bacterium. Biochemistry. 1968 Jun;7(6):2249–2256. doi: 10.1021/bi00846a030. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., INGRAM M. The freezing points of bacterial cells in relation to halophilism. J Gen Microbiol. 1959 Feb;20(1):27–31. doi: 10.1099/00221287-20-1-27. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchow E., Douglas H. C. RHODOMICROBIUM VANNIELII, A NEW PHOTOHETEROTROPHIC BACTERIUM. J Bacteriol. 1949 Oct;58(4):409–416. doi: 10.1128/jb.58.4.409-416.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman M., Swinton D., Schiff J. A., Epstein H. T., Zeldin B. Deoxyribonucleic Acid of the blue-green algae (cyanophyta). Bacteriol Rev. 1967 Dec;31(4):315–331. doi: 10.1128/br.31.4.315-331.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI M., GOTO K., FUJIMOTO M., NAMIKI O., KIKUCHI G. EFFECT OF INHIBITORS OF NUCLEIC ACID AND PROTEIN SYNTHESES ON THE INDUCED SYNTHESES OF BACTERIOCHLOROPHYLL AND DELTA-AMINOLEVULINIC ACID SYNTHETASE BY RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1965 Jan 11;95:94–110. doi: 10.1016/0005-2787(65)90215-7. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L. R. An index to deoxyribonucleic acid base compositions of bacterial species. J Gen Microbiol. 1966 Sep;44(3):419–437. doi: 10.1099/00221287-44-3-419. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSHI J. G., GUILD W. R., HANDLER P. The presence of two species of DNA in some halobacteria. J Mol Biol. 1963 Jan;6:34–38. doi: 10.1016/s0022-2836(63)80079-0. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Heterogeneity in deoxyribonucleic acids. I. Dependence on composition of the configurational stability of deoxyribonucleic acids. Nature. 1959 May 23;183(4673):1427–1429. doi: 10.1038/1831427a0. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- MARTINEZSEGOVIA Z. M., SOKOL F., GRAVES I. L., ACKERMANN W. W. SOME PROPERTIES OF NUCLEIC ACIDS EXTRACTED WITH PHENOL. Biochim Biophys Acta. 1965 Feb 8;95:329–340. doi: 10.1016/0005-2787(65)90497-1. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HARADA K., KAMEDA M. Elimination of transmissible drug-resistance by treatment with acriflavin. Nature. 1961 Mar 18;189:947–947. doi: 10.1038/189947a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Turnock G., Forchhammer J. The synthesis and function of ribosomes in a new mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jan;57(1):141–147. doi: 10.1073/pnas.57.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Base sequence homology and renaturation studies of the deoxyribonucleic acid of extremely halophilic bacteria. J Bacteriol. 1969 Jul;99(1):255–262. doi: 10.1128/jb.99.1.255-262.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Comparative study of ribosomal ribonucleic acid cistrons in enterobacteria and myxobacteria. J Bacteriol. 1967 Oct;94(4):1066–1074. doi: 10.1128/jb.94.4.1066-1074.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. C., Sistrom W. R. The isolation and preliminary characterization of a halophilic photosynthetic bacterium. Arch Mikrobiol. 1967;59(1):255–268. doi: 10.1007/BF00406339. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- SUEOKA N., MARMUR J., DOTY P., 2nd Dependence of the density of deoxyribonucleic acids on guanine-cytosine content. Nature. 1959 May 23;183(4673):1429–1431. doi: 10.1038/1831429a0. [DOI] [PubMed] [Google Scholar]
- Schumaker V. N., Wagnild J. Zone centrifugation in a cesium chloride density gradient caused by temperature change. Biophys J. 1965 Nov;5(6):947–964. doi: 10.1016/S0006-3495(65)86761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y., Gibson J. Satellite DNA in photosynthetic bacteria. Biochem Biophys Res Commun. 1966 Aug 23;24(4):549–553. doi: 10.1016/0006-291x(66)90355-x. [DOI] [PubMed] [Google Scholar]



