Summary
The establishment and stability of cell fates during development depend on the integration of multiple signals, which ultimately modulate specific patterns of gene expression. While there is ample evidence for this integration at the level of gene regulatory sequences, little is known about its operation at other levels of cellular activity. Wnt and Notch signalling are important elements of the circuitry that regulates gene expression in development and disease. Genetic analysis has suggested that in addition to convergence on the transcription of specific genes, there are modulatory cross regulatory interactions between these signalling pathways. Here we report that the nodal point of these interactions is an activity of Notch which regulates the activity and the amount of the active/oncogenic form of Armadillo/ß-catenin. This activity of Notch is independent of that induced upon cleavage of its intracellular domain and which mediates transcription through Su(H)/CBF1. The modulatory function of Notch described here, contributes to the establishment of a robust threshold for Wnt signalling which is likely to play important roles in both normal and pathological situations
INTRODUCTION
The Drosophila Notch gene encodes a member of a family of single transmembrane receptors that play a central role in the assignation of cell fates during development (Artavanis-Tsakonas et al., 1999; Kopan, 2002). The extracellular domain of Notch is composed of an array of EGF-like repeats that are involved in ligand binding and three cysteine rich domains (LNR) required for signal transduction (Brennan et al., 1999b; Lawrence et al., 2000; Lieber et al., 1993; Rebay et al., 1991). The intracellular domain is the signalling moiety of the receptor and its most prominent structural feature is a group of six cdc10/Ankryn (ANK) repeats that are involved in a variety of molecular interactions (Kopan et al., 1994; Lieber et al., 1993; Rebay et al., 1993; Struhl et al., 1993). Upon binding its ligand Delta, a member of the DSL family, Notch undergoes a sequence of proteolytic cleavage events that release the intracellular domain (NICD) from the membrane (Schroeter et al., 1998; Schweisguth and Lecourtois, 1998; Struhl and Adachi, 1998). NICD then enters the nucleus where it interacts with Suppressor of Hairless (Su(H)/CBF1) (Artavanis-Tsakonas et al., 1999; Barolo et al., 2002; Kidd et al., 1998; Kopan, 2002) and regulates the transcription of specific targets (Artavanis-Tsakonas et al., 1999; Kopan, 2002). This signalling event is used in some inductive events but more importantly in multiple binary cell fate decisions in which Notch signalling favours one of two alternative fates by suppressing the onset of the genetic programme that would lead to the other fate (Artavanis-Tsakonas et al., 1999; Kopan, 2002).
There is evidence that Notch can also signal in a Su(H) independent manner (Endo et al., 2002; Endo et al., 2003; Martinez Arias et al., 2002). A number of experiments in Drosophila indicate that this alternative pathway modulates signalling by Wingless, a member of the Wnt family of signalling molecules (Martinez Arias et al., 2002). Loss of function of Notch, but not of Delta or of Su(H), can bypass loss of function of wingless, or of dishevelled, a gene which encodes a core element in the transduction of the Wnt signal (Brennan et al., 1999a; Lawrence et al., 2001). This suggests that Notch can downregulate Wnt signalling in a Su(H)-independent manner, a notion reinforced by the existence of gain of function mutations in Notch which antagonise Wingless signalling (Brennan et al., 1999b; Martinez Arias et al., 2002; Ramain et al., 2001). Consistent with these observations, removal of Notch1 in the skin leads to tumours associated with Wnt signalling and with high levels of the nuclear form of ß-catenin (Nicolas et al., 2003). There is some evidence from different systems that Deltex, a ubiquitin ligase, is involved in the Su(H) independent event and perhaps in the modulation of Wingless signalling. However, even though the interaction between Notch and Wingless signalling is well established at the genetic level its molecular mechanism remains.
It is generally accepted that the key parameter of Wnt signalling is the stability and precise intracellular location of a soluble pool of Armadillo/ß-catenin (Arm/ß-cat) (Gottardi and Gumbiner, 2001). In the absence of Wnt this pool interacts with a destruction complex assembled around the scaffolding protein Axin, where it is phosphorylated by Shaggy/GSK3ß and degraded via the proteasome. Wnt acting through the Frizzled and Arrow/LRP receptors activates the cytoplasmic adaptor protein Dishevelled which, in a poorly understood manner leads to the inactivation of the destruction complex and allows the accumulation of a hypophosphorylated form of Armadillo/ß-catenin. This form then enters the nucleus where it interacts with members of the TCF family of transcription factors to influence the transcriptional state of the cell (Tolwinski and Wieschaus, 2004a). While the central role of Armadillo/ß-catenin as a nuclear effector of Wnt signalling is well established, the mechanism whereby it is activated remains open to discussion (Giles et al., 2003; Tolwinski and Wieschaus, 2004b). The well established notion that the key regulatory event is the activity of Shaggy/GSK3ß has recently be revised with the observation that Axin has effects on Wnt signalling that are independent of Shaggy/GSK3ß (Tolwinski et al., 2003). These observations have suggested that the central event in the activation of Armadillo/ß-catenin is the activity of Axin.
Here we analyse the molecular nature of the interactions between Notch and Wingless signalling in Drosophila and between mouse Notch1 and ß-catenin. Our experiments show that Notch modulates Wingless signalling by regulating the stability and activity of Armadillo and that there is a pool of Armadillo that is associated with Notch in vivo. This activity of Notch is independent of that which mediates CBF1/Su(H) dependent signalling. Most importantly we also show that Notch has the ability to downregulate the activity of an oncogenic form of Armadillo. Our results indicate that Notch plays an important role in the modulation of Armadillo function in vivo. We discuss the implications of our findings for current models of Wnt signalling during normal development and carcinogenesis.
Materials and Methods
Immunohistochemistry and genetic analysis
Imaginal wing disc were dissected from third instar larvae with in fixed solution[4% paraformaldehyde in BBS with 1mM CaCl2]. Discs were fixed for 30 minutes, and then immuno-stained with indicated antibodies in BBS [50 mM BES, 280 mM NaCl, 1.5 mM Na2HPO4.2H2O]+ 0.1% Triton X100, 0.5% BSA 1mM CaCl2] using standard antibody staining protocols (Antibodies anti-Armadillo N27A1, anti-MYC 9E10, anti-Distalless, anti-Senseless). Discs were mounted in Vecta sheild and viewed using a Confocal microscope (Note. same gain used in each figure set).
The activity of various genes has been eliminated by generating clones of mutant cells in an otherwise heterozygous background through the FLP recombinase system as described before (Klein et al., 2000). The clones were induced over the domain of expression of the ptc gene and were identified by the loss of GFP. The following stocks were used as the source of the mutations in the experiments: Df(1)N81k v [FRT101 w+]/FM6: sggD127 ; N55e11 [FRT101 w+]/FM6f and wa sggm11 sn3 [FRT101 w+]/FM6f. All are null alleles of the different genes. Each of these stocks was outcrossed to yw ubiqGFPx1 [FRT101 w+]; ptcGAL4; UASFLP A101lacZ/SM6a^TM6B. As a result clones were induced continuously throughout development over the domain of ptc expression. In some of the experiments we induced the ectopic expression of particular forms of Notch or Armadillo over the domain of ptc. This was achieved with the stocks: wa sggm11 [FRT101 w+]/FM7c; UASTNotch and Df(1)N81k v [FRT101 w+]/FM6;; UASArmadillo. TNotch (Seugnet et al., 1997) and UAS Armadillo (Seugnet et al., 1997) have been described before.
Analysis of Armadillo protein levels
Wing discs from 3rd instar larvae expressing Armadillos10, TNotch, FLNotch or NICD under the control of dppGAL4 were lysed in 2X laemmli buffer (Harlow and Lane, 1988). Proteins were separated by 8% SDS-PAGE, the equivalent of 5 wing discs were loaded per lane. Western blot analysis for Armadillo (N2 7A1), Armadillos10 (anti-MYC, 9E10) and Tubulin (E7) was performed.
Cell based reporter assays
RNAi: Wing imaginal disc-derived Clone 8 cells (Peel et al., 1990) were used in the RNAi experiment. Transfections were performed in triplicate in 96-well plates using Effectene transfection reagent (Qiagen). The ratio of Luciferase reporter (Top12X-HS-Luciferase; R. D and N. P, Manuscript in preparation), normalisation vector (Renilla luciferase) was 1:1, 50ng of total DNA added per well. dsRNAs were synthesised using in vitro transcription from PCR product templates which have T7 polymerase binding sites as linkers (as described in (Boutros et al., 2004)). 80ng of dsRNA was added to each transfection reaction along with the total DNA. The amount of Firefly and Renilla luciferase was measured 4 days after transfection using Dual-Glo luciferase reagent (Promega). Data is normalised with respect to Renilla luciferase and presented and relative light units (RLU), all data presented represents at least 3 independent experiments. Reporter assays in Insect Cells: SL2 (Nagao et al., 1996) and S2R+ (Yanagawa et al., 1998) cells were used in gain of function experiments. Transfections were performed in triplicate in 96-well plates using Effectene transfection reagent (Qiagen). The ratio of Luciferase reporter (Top12X-HS-Luciferase), normalization vector (Renilla luciferase), Inducer (pPac-S37Aßcat (Schweizer and Varmus, 2003)) DNA was 1:1:2, the remaining DNA was composed of variable amounts of pPACTN and pPAC with a total amount 200ng DNA added per well. The amount of Firefly and Renilla luciferase was measured 4 days after transfection using Dual-Glo luciferase reagent (Promega). Data is normalised with respect to Renilla luciferase and presented and relative light units (RLU), all data presented represents at least 3 independent experiments. Reporter assay in Mammalian cells; plasmid constructs: Two Notch1(Nye et al., 1994) constructs bearing extracellular deletions were generated, LNR-N1 lacks amino acids 19-1654, which removes the EGF-like and LNR repeats and the encoded protein should be identical to the transmembrane and intracellular fragment produced following für in cleavage at the S1 site. ΔN-N1 lacks amino acids 19-1710, which removes all but 13 amino acids of the extracellular domain and the encoded protein should be identical to the protein produced upon cleavage at the S2 site during ligand induced signalling. cDNAs encoding these proteins were sub-cloned into pSecTag2 (Invitrogen) to replace the endogenous signal peptide with the Ig κ-chain signal peptide. c-Myc and poly-histidine epitopes were introduced immediately downstream of the signal cleavage site to aid detection of the expressed proteins. Plasmids encoding mouse Wnt1 (Shimizu et al., 1997), Xenopus Dishevelled (Sokol, 1996)and Xenopus ß-catenin (Kypta et al., 1996) have been described previously. The pCS2+ plasmid encoding the Lef1-VP16 fusion protein was obtained from Dr. R. Kemler, Max-Planck Institut fur Immunbiologie and the CBF Reporter was provided by Dr G. McKenzie, Lorantis Ltd. Luciferase assays were carried out as follows. Cells were seeded in 24-well plates at 1×105 cells/well. 20 hrs later, the cells were transfected by using the calcium phosphate co-precipitation method with a plasmid cocktail containing 0.22μg of DNA (including 50ng pTOPFLASH or CBF1 luciferase reporter, and 20ng pRL-CMV). All transfections were done in triplicate and the cells were incubated with the plasmid cocktail overnight. Lysates were prepared 48 hrs after transfection, and Firefly and Renilla luciferase activities in 5 μl of lysate were measured with the Dual Luciferase Reagent kit (Promega).
Immunoprecipitation experiments
Wild type embryos were dechorionated and lysed in RIPA or NP-40 buffer (Harlow and Lane, 1988). Each immunoprecipitation reaction contained the equivalent of 5μl packed volume of embryos homogenised in 250 μl lysis buffer. Notch proteins were immunoprecipitated using either 20 μl anti-NCID sheep antiserum or 50 μl anti-Notch (C17.9C6) and 20 μl Protein-G sepharose. Armadillo proteins were immunoprecipitated using 10 μl anti-Armadillo rabbit antiserum and Protein-A sepharose. In each immunoprecipitation experiment control reactions of either Protein-G, Protein-A or anti-GFP rabbit antiserum with Protein-A were undertaken. Immune complexes were released by boiling in 60 μl laemmli buffer and separated by 8 % SDS-PAGE, 20 μl immunoprecipitate per lane. Proteins were detected by Western blot using anti-Armadillo (N27A1), anti-Notch (C17.9C6 or sheep antiserum) or anti-Dishevelled (rabbit antiserum).
RESULTS
Notch modulates wingless signalling by regulating the activity of Armadillo
A soluble form of the intracellular domain of Notch, NICD, acts as an activated Notch receptor and provides constitutive Su(H) dependent Notch signalling (reviewed in (Schweisguth, 2004)). On the other hand a chimera between the extracellular and transmembrane domains of the receptor tyrosine kinase (RTK) Torso and the intracellular domain of Notch (TNotch) prevents the cleavage of Notch and the translocation of its intracellular domain to the nucleus (Struhl and Adachi, 2000). However, this chimeric molecule is still capable of signalling as reflected by the loss of neural precursors during neurogenesis ((Seugnet et al., 1997; Zecchini et al., 1999) and A. Martinez Arias unpublished). This signalling event is likely to be independent of Su(H) because while NICD and full length Notch are able to activate transcription of either a Su(H) reporter in vivo (Furriols and Bray, 2001) or the Notch target gene Wingless (Diaz-Benjumea and Cohen, 1995; Klein and Martinez Arias, 1998), TNotch is unable to do so (A. Martinez Arias unpublished).
The inputs of Notch and Wingless signalling on the development of the wing are well characterised (Klein and Martinez Arias, 1998; Klein and Martinez Arias, 1999; Martinez Arias, 2003) and we have taken advantage of this to analyse how Notch modulates Wingless signalling. Notch and Wingless signalling cooperate in the development of the wing and in the case of Notch the effects are mediated by NICD. To test if the cleavage independent function of Notch modulates Wingless signalling, we have expressed NICD and TNotch at the same time that we activate Wingless signalling either with ectopic expression of Wingless or of a constitutive active form of Armadillo, Armadillos10. This form of Armadillo lacks the Shaggy/GSK3ß phosphorylation sites and provides Wingless independent signalling by escaping degradation by the Axin based destruction complex (Pai et al., 1997). In these experiments ectopic signalling is induced by expressing the active component along the anterior/posterior (AP) boundary of the developing wing disc. As shown before, expression of either Wingless or Armadillos10 along the AP boundary results in an expansion of the hinge region and the occasional appearance of extra wing tissue off the notum (Klein and Martinez Arias, 1998) (Fig.1C). However the effects of the intracellular domain of Notch depend on its molecular disposition. Expression of NICD along the AP boundary induces the appearance of an ectopic wing margin and promotes the growth of the wing (Diaz-Benjumea and Cohen, 1995; Klein and Martinez Arias, 1998), while expression of TNotch leads to a slight reduction in the overall size of the wing pouch region of the disc (Fig.1B). In the developing wing, co-expression of NICD with either Wingless or Armadillos10 leads to a synergistic effect of extra growth of the wing tissue (Klein and Martinez Arias, 1998). In contrast to NICD, TNotch is very effective in suppressing the effects of ectopic expression of Wingless (Fig.S1) and, surprisingly, also of Armadillos10 (Fig.1D and S1).
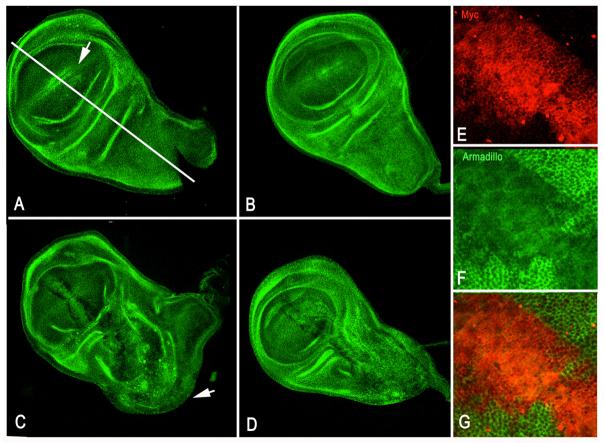
Figure 1. Notch modulates the activity of an activated form of Armadillo.
Third larval instar wing discs stained for Armadillo and expressing different signalling molecules. (A) Apical section through a wild type wing disc stained for Armadillo. Notice the elevated levels of Armadillo protein around the Dorsal/Ventral (DV) boundary (arrow) which coincide with high levels of Wingless signalling. The white stripe indicates the approximate domain of dpp and ptc expression, just anterior to the AP compartment boundary, which demarcates the regions of ectopic expression in our experimental situations. (B) Apical section through a disc expressing TNotch under the control of dppGAL4. This has few consequences other than slight differences in the pattern of endogenous Armadillo, particularly at the DV boundary. (C) Wing disc expressing (Pai et al., 1997)under the control of dppGAL4 . The epitope recognised by the monoclonal antibody N27A1 is not present in ArmadilloS10 and therefore the Armadillo protein shown is the endogenous one. Notice that the domain of expression of ArmadilloS10 is demarcated by changes in the concentration and subcellular location of the endogenous Armadillo and also in an alteration of growth in the notum (arrowhead and see (Pai et al., 1997)). The focal plane shown here is about the middle of the epithelium and displays the increase in the cytoplasmic concentration of the endogenous Armadillo. (D) Co-expression of TNotch with ArmadilloS10 suppresses significantly the effects of ArmadilloS10 both on the shape of the disc as well as on the alterations in the distribution of the endogenous Armadillo (compare to C, similar focal plane, and see also S1). (E-G) Confocal images through a wing disc expressing ArmadilloS10 under the control of dppGAL4 at the level of the adherens junctions showing that ArmadilloS10 displaces endogenous Armadillo from this region of the cell; posterior to the right and anterior to the left. (E) ArmadilloS10 is tagged with the Myc epitope (Pai et al., 1997) and therefore can be specifically detected with an anti-myc antibody. Notice its expression in a stripe that corresponds to the domain of dpp expression and its association with the adherens junctions. (F) The endogenous Armadillo is detected by the N27A1 antibody. As shown the endogenous Armadillo is excluded from the adherens junctions over the domain of ArmadilloS10. The shadows in the picture correspond to the cells from the peripodial membrane which are right against the apical side of the epithelia of the disc. (G) Merge of E and F showing the cell autonomous effects of ArmadilloS10 on endogenous Armadillo.
Since Armadillos10 is thought to provide Wingless signalling constitutively (Pai et al., 1997) and the expression of TNotch does not affect the expression of Wingless in the third instar discs (Fig.S1), these results argue that a Su(H)-independent Notch activity modulates Wingless signalling by targeting the activity of Armadillo. To test this further, we analysed the effects of TNotch on the ability of Armadillos10 to induce expression of Wingless target genes, Distalless (Dll) a low threshold target of Wingless, and the proneural gene senseless (sens), which like other proneural genes, provides a high threshold target (Zecca et al., 1996). Both are elevated and ectopic in the presence of Armadillos10, in both cases TNotch markedly suppresses this effect (Fig.2).
Figure 2. Notch suppresses the activity of ArmadilloS10.
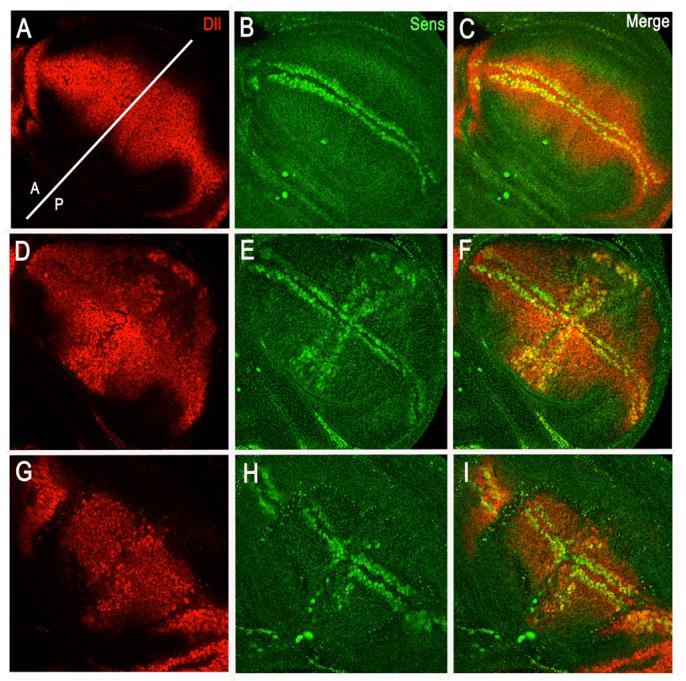
Details of the wing pouch region of third larval instar wing discs showing the response of high (Senseless, Sens) and low (Distalless, Dll) threshold targets of Wingless signalling to normal or ectopic activity of Armadillo. All antibody stains against the different gene products. (A-C) Wild type. (A) Distalless expression in the wing pouch of a wild type disc. Notice a slight elevation of the expression at the DV boundary. The white stripe indicates the approximate domain of dpp expression, just anterior to the AP compartment boundary, which demarcates the regions of ectopic expression in our experimental situations. (B) Expression of the proneural protein Senseless highlighting neural precursors which develop on either side of the DV boundary in response to high levels of Wingless signalling (Couso et al., 1993). (C) Merge of the images in A and B. (D-F) Wing pouch of a disc expressing ArmadilloS10 under the control of dppGAL4. (D) Distalless expression is now elevated and expanded over the AP boundary in a domain that corresponds to that of ArmadilloS10 (compare to A). (E) Senseless can be detected over a new domain along the AP boundary which corresponds to the domain of ArmadilloS10 expression. (F) Merge of D and E showing how the ectopic expression of Senseless coincides with the elevated levels of Distalless. (G-I) Wing pouch of a disc expressing ArmadilloS10 together with TNotch under the control of dppGAL4. (G) The effects of ArmadilloS10 on Distalless are suppressed by TNotch and a reduction of wild type levels can also be observed. (H) The ectopic expression of Senseless induced by ArmadilloS10 is suppressed by TNotch. Notice also the reduction in the endogenous expression (arrow) over the domain of TNotch expression. (I) Merge of G and H.
To test whether the effects that we observe are restricted to the developing wing, we have monitored the effects of TNotch on the cuticle pattern of the first instar larva. In the wild type each segment contains an anterior region decorated with denticles and a ‘naked’ posterior region, devoid of denticles (Pai et al., 1997) (Fig.3A). The extent of the ‘naked’ region depends on the level of Wingless signalling, and ubiquitous Wingless signalling associated with strong expression of Armadillos10 results in cuticles all devoid of denticles (Pai et al., 1997). By modulating the levels of expression of Armadillos10 it is possible to modulate the extent of denticle loss: weak expression leads to a patchy loss of denticles (Fig.3B) in contrast to strong expression which results in ventral cuticles completely devoid of denticles (Fig.3C). Expression of TNotch modulates the effects that Armadillos10 has on the pattern of the cuticle: while strong effects of Armadillos10 are often suppressed (Fig.3D), weak effects are very easily suppressed (Fig.3E). This observation confirms that Notch exerts a negative modulation on Wnt signalling and suggests that this might be a general phenomenon.
Figure 3. Modulation of Wnt signalling by Notch during the patterning of the larval cuticle.
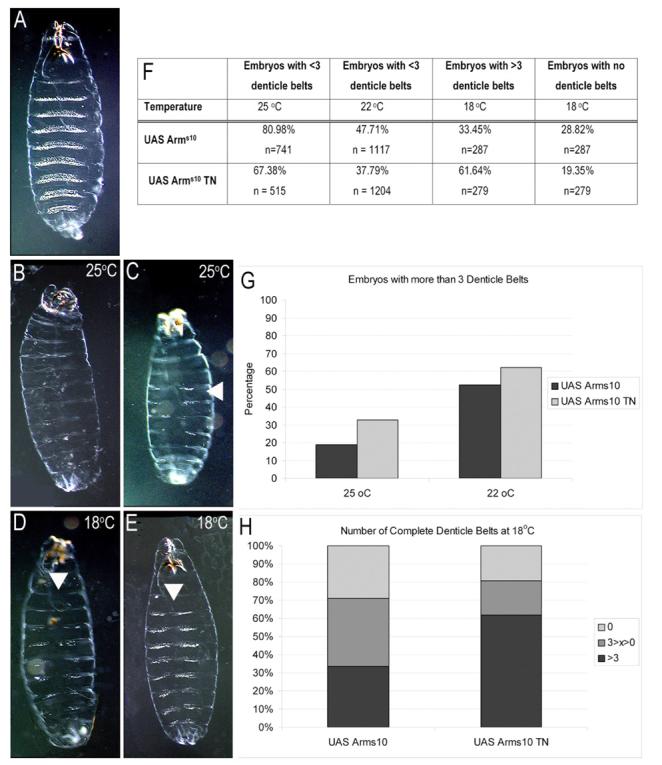
(A) Wild type pattern of the larval cuticle at the end of embryogenesis. The cuticle exhibits reiterated thick sets of denticles that demarcate the anterior part of each segment and are very prominent in the abdominal region; the posterior part of each segment is devoid of denticles and this phenotype requires Wingless signalling. (B-E) Ventral cuticles of larvae expressing UAS Arms10 (B, D), or UAS Arms10 together with UAS TNotch (C, E), under the control of armGAL4 at different temperatures. The activity of GAL4 is temperature dependent and elicits more expression of the genes under GAL4 UAS control at the higher temperature; this can be seen in the penetrance of the phenotypes (F-H). (B-D) Cuticles representative of the phenotypes caused by expression of Arms10 (B) or Arms10 together with TNotch (C) at 25°C. At this temperature GAL4 is very active and Arms10 consistently elicits the naked cuticle fate (see also F). TNotch suppresses the effects of Arms10 and allows the development of denticles (arrow). The effect of TNotch is more pronounced at 22°C (D, E), when the effects of Arms10 are weaker. At 18°C most embryos expressing Arms10 develop some denticles, however they tend not to cross the midline (see arrow in D). At this temperature the effects of TNotch on Arms10 are manifested in the appearance of denticles across the midline (see arrow in E). (F-H) Quantitative analysis of the effects of TNotch on Arms10 at different temperatures (25°C, 22°C and 18°C). (F and G) At 25°C and 22°C the effects of Arms10 were assessed by the number of embryos with 3 or less segments with denticles regardless of whether they cross the midline or not. Notice that at both temperatures (25°C, 22°C) embryos that express TNotch have more segments with denticles. (F and H) At 18°C most embryos expressing Arms10 have segments with denticles due to the low expression of Arms10. In this case we evaluated the number of embryos with denticle belts i.e. embryos in which the denticles cross the midline. As in( G), we chose 3 as an arbitrary threshold for our quantification. In this instance we also counted the number of embryos with naked cuticle. TNotch can suppress the effects of Arms10.
Altogether these observations suggest that there is an activity of Notch, independent of Su(H), which modulates the Wingless signalling pathway at or below the level of Armadillo.
Torso-Notch modulates the levels of Armadillo
The effects of Notch on the activity of Armadillos10 could be due to a squelching of GAL4 by the UAS TNotch construct reducing the expression of other constructs co-expressed with it. However in situ experiments demonstrate that UAS TNotch transcription does not affect UAS Armadillos10 expression (P. Hayward unpublished). This suggests that the effects of Notch on the activity of Armadillo result either from a parallel input on Wingless target gene expression or from an effect on Armadillo itself. In order to test this we have analysed the effects of Notch on the levels, state and localisation of the Armadillo protein.
In the epithelium of the wing disc, Armadillo is preferentially localised at the level of the adherens junctions (Fig.1E, F) and exists in at least two different phosphorylation states (Fig. 4E) that have been correlated with function (Peifer et al., 1994a): a hypophosphorylated form which has been associated with nuclear activity (Staal et al., 2002) and a hyperphosphorylated form which is predominantly restricted to the adherens junctions (Peifer et al., 1994b). In our experiments, when Armadillos10 is expressed it becomes preferentially localised to the adherens junctions (Fig 1E and A. Martinez Arias unpublished) we do not observe an accumulation of Armadillo in the nuclei under these fixing conditions. The expression of Armadillos10 has a significant effect on the endogenous Armadillo, which is displaced from the adherens junctions and accumulates in the cytoplasm (Fig.1C, F). In Western blots this is translated into an overall rise in endogenous Armadillo levels and is correlated with an increase in the proportion of the hypophosphorylated form (Fig.4E, F). These effects are likely to be associated with the enhanced stability of Armadillos10and its ability to interact with the components of the Armadillo destruction complex (Cox et al., 1999; Pai et al., 1997) e.g. Axin and APC, which will result in a titration of their activity and a resultant stability of the endogenous form of Armadillo. Similar arguments have been invoked before for membrane-tethered forms of Armadillo (Tolwinski and Wieschaus, 2001), however Armadillos10 is not membrane tethered.
Figure 4. Notch affects the levels of Armadillo and of ArmadilloS10.
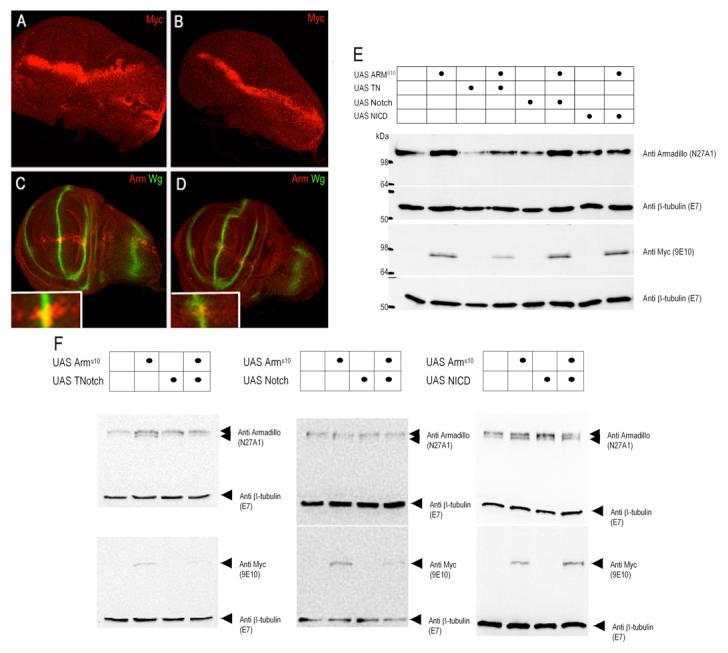
Effects of TNotch on the stability of ArmadilloS10 and wild type Armadillo under the control of dppGAL4 in third instar wing discs (A-D) and Western blots analysing the effects of Notch, TNotch and NICD on the concentration of Armadillo and Armadillos10 in third instar wing discs (E and F). The experiments shown here were performed at 22°C. The discs are shown with anterior down and posterior top. (A) Third larval instar wing disc showing the expression of ArmadilloS10 in a stripe along the AP boundary (dppGAL4) revealed with an anti-Myc antibody. Notice the stability of the expression relative to wild type Armadillo in (C). (B) Expression of TNotch with ArmadilloS10 reduces the overall amount of ArmadilloS10. (C) Expression of wild type Armadillo under the same conditions as ArmadilloS10 in (A). Armadillo (red) is very unstable, mostly cytoplasmic and is only stabilised in the presence of Wingless (green and see Figure S2). (D) TNotch destabilises the overexpressed wild type Armadillo and antagonises Wingless signalling. Notice that the ectopic Armadillo has been almost completely eliminated except for a small amount in the very neighbourhood of the Wingless expressing cells (see also inset) and that this is not associated with a loss of Wingless expression. The stripe of Wingless expression in the DV boundary (green) is slightly broadened over the domain of expression of TNotch, a phenotype characteristic of loss of Wingless signalling at the beginning of the third instar (Rulifson et al., 1996). (E and F) Western blots showing the concentration of endogenous Armadillo and ArmadilloS10 in the presence or absence of various forms of Notch. Levels of endogenous Armadillo (detected with N27A1), Armadillos10 (detected with anti-Myc) and tubulin (detected with E7, acts as loading control) are shown. Expression of Armadillos10 and Notch molecules was under the control of dppGal4 in (E) or C5Gal4 in (F). In the presence of Armadillos10 we observe an elevation of endogenous Armadillo levels (E) or an increase in the hypophosphorylated form (lower band of Armadillo doublet, (F)) compared to wild type. Expression of TNotch under the control of dppGal4 results in a marked decrease in the amounts of both Armadillo and Armadillos10 , in this gel Armadillo protein is visualised as one band due to insufficient separation (E), whereas expression of TNotch under the control of C5Gal4 results in a reduction in levels of the hypophosphorylated form of endogenous Armadillo and also of Armadillos10 (F). The effects of full length Notch (Notch) are less marked, but when under the control of dppGal4 Notch expression results in a decrease of endogenous Armadillo (E); and a reduction in the level of Armadillos10 is apparent when Notch expression is under the control of C5Gal4 (F). Expression of NICD results in small increases in the amounts of Armadillo or Armadillos10 (E and F) in our experimental conditions, this is probably due to the induction of Wingless expression by NICD.
To provide a measure of the effects, of Armadillos10 we have performed Western blot analysis on its steady state levels as well as those of endogenous Armadillo, in the presence or absence of various forms of the Notch receptor. These experiments were performed with three different Gal4 lines which direct expression of effector genes in different but overlapping domains: the whole wing pouch (C5Gal4, Fig.4E), the Hh signalling domain (dppGal4, Fig,4F)) and a domain around the DV boundary (C96Gal4, P. Hayward and P. Sanders unpublished). Consistent with what we observe in the disc epithelium, expression of Armadillos10 elevates the overall levels of endogenous Armadillo with a pronounced increase in the hypophosphorylated form (lower band of doublet, see Fig.4F). Expression of both TNotch and full length Notch can reduce the levels of all forms of Armadillo, but the extent depends on the expression domain.
Expression of TNotch under the control of dppGal4 results in a large reduction in both endogenous Armadillo and Armadillos10, whereas the effects of full length Notch are limited to endogenous Armadillo. Under the control of C5Gal4 expression of both TNotch and full length Notch regulate the levels of Armadillos10, under these conditions the hypophosphorylated form of endogenous Armadillo is also reduced in the presence of TNotch. In contrast, NICD expression results in an increased accumulation of Armadillos10 and no obvious affect on endogenous Armadillo levels (Fig.4E, F). This effect is likely to be due to the ectopic expression of Wingless induced by NICD (Diaz-Benjumea and Cohen, 1995) which will lead to an increased stabilisation of Armadillo.
To test further the effects of Notch on Armadillo we over-expressed full length Armadillo together with TNotch. When Armadillo is overexpressed on its own, it accumulates to very high levels in the cytoplasm of the cells (Marygold and Vincent, 2003) in a manner that is strictly dependent on Wingless signalling and other less characterised factors (Fig.S2). This accumulation is significantly reduced in the presence of TNotch (Fig.4C, D).
The data we have presented here demonstrate that in the imaginal wing disc the activity and levels of both Armadillo, and Armadillos10, a form that mimics oncogenic forms of ß-catenin, are the subject of regulation by the Notch receptor.
Notch modulates the transcriptional activity of Armadillo
The results that we have presented indicate a regulatory effect of Notch on the concentration and transcriptional effects of an activated form of Armadillo in vivo. Although our observations suggest a direct effect of Notch on the activity of Armadillo, the complexity of the in vivo regulatory networks could conceivably create situations that would yield the observed effects indirectly. To rule this out and analyse the interaction further we studied the effects of Notch loss and gain of function on Wnt signalling in Drosophila cells in culture by measuring the effects of Notch on the activity of a Wnt reporter (TOP12).
If gain of function of Notch suppresses Wnt signalling in the wing disc, we asked what would happen to Wnt signalling in the absence of Notch. Earlier experiments in vivo have shown that removal of Notch results in ectopic activity of a Wnt reporter (Lawrence et al., 2001). Here we have tested this in culture by taking advantage of Clone8 cells, cl8, a diploid cell population derived from wing imaginal discs that have been used for a variety of assays of Wnt activity (van Leeuwen et al., 1994). The TOP12 reporter is functional in these cells and is activated by Wnt signalling in a dose dependent manner (R. Dasgupta unpublished ). There is no detectable activity of the reporter in these cells but surprisingly, reduction of Notch signalling by targeted RNA interference (RNAi) of the Notch gene results in activation of the reporter to significant levels (Fig.5B). In these experiments, four different dsRNA molecules directed against the coding region of the intracellular domain of Notch resulted in a quantitatively different but qualitatively similar effect (P. Hayward unpublished). These results confirm that Notch exerts a negative effect on Wnt signalling.
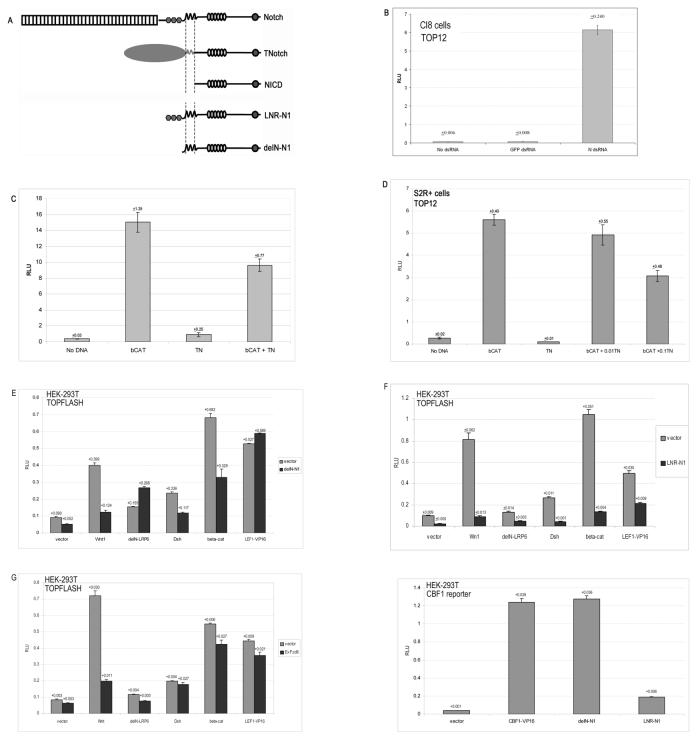
Figure 5. Notch modulates Wnt pathway transcriptional activity in both Drosophila and Vertebrate cells.
(A)Diagram of the different Notch molecules used in this study. (B) Loss of Notch function in Drosophila clone 8 (cl8) cells results in ectopic activation of Wnt signalling. Cells were transfected with the TOP12 reporter and a Renilla luciferase standard in the presence of no dsRNA, GFP dsRNA or Notch dsRNA, as shown. Ectopic activation of the Wnt signalling pathway was observed in cl8 cells in the presence of Notch dsRNA (103.6 fold activation compared to treatment with no dsRNA) but not GFP dsRNA (1.1 fold activation). (C , D) Gain of function of Notch in Drosophila cells results in repression of ectopically activated Wnt signalling. SL2 cells (C), or S2R+ cells (D) were transfected with the TOP12 reporter and Renilla standard, Wnt signalling was induced with an oncogenic form of ß-catenin, S37A β-catenin (Schweizer and Varmus, 2003). In the presence of a membrane tethered form of Notch (TNotch) the level of ectopic Wnt signalling was significantly reduced (C, D). (E-H) Gain of function of mouse Notch 1 results in repression of ectopic Wnt signalling induced by Wnt 1, Dsh and β-catenin in HEK-293T cells. Cells were transfected with the TOPFLASH reporter and a Renilla standard. Ectopic Wnt signalling was induced with Wnt1, delN-LRP6), Dishevelled, β-catenin or LEF1-VP16. In these assays two forms of Notch 1 were utilised; delN-N1 and LNR-N1(see A). DelN-N1 lacks the EGF and LN repeats and cleaves spontaneously to release the NICD domain of Notch1 as shown by the strong activation of the CBF1 reporter in HEK-293T cells (H), activation of the CBF1 reporter is approximately equivalent to that generated by an activated form of CBF1, CBF1-VP16. LNR-N1 is a membrane tethered form of N1 which lacks the EGF repeats but retains the LN repeats, that rarely cleaves as shown by the weak activation of the CBF1 reporter in HEK-293T cells (H and see also (Mumm et al., 2000)). In these experiments, canonical inhibition of Wnt signalling was effected by expression of ExFz8, which inhibits Wnt signalling by titrating Wnt (Brennan et al., 2004) but has no direct effect on the intracellular activation of the pathway. Small effects of ExFz8 on the endogenous Wnt signalling are visible, such effects have previously been reported by Suzuki et al.(Suzuki et al., 2004) (G). Both forms of Notch are capable of significantly repressing ectopic Wnt signalling induced by Wnt1, Dsh, and β-catenin (E and F), LNR-N1 effects also extended to ectopic Wnt signalling induced by delN-LRP6 and LEF1-VP16. On the other hand, ExFz8 repressed ectopic Wnt signalling induced by Wnt1 but had no effect intracellular mediators of Wnt signalling (G).
To study the effects of activation of Notch signalling we made use of SL2 cells and S2R+ cells (both derived from the Drosophila S2 cell line), the former lack DFz2 (Nagao et al., 1996; Yanagawa et al., 1998). In these cells, transfection of an oncogenic form of vertebrate ß-catenin, S37A-ß-catenin, which signals constitutively, results in a robust and significant activity of the TOP12 reporter (Fig.5C, D). Co-transfection of TNotch or full length Notch with S37A-ß-catenin, results in a decrease in the activity of the reporter (Fig.5C, D and P. Hayward unpublished). The reduction in activity is related to the amount of Notch molecules in the assay (Fig 5D ). In cl8 cells, TNotch and full length Notch exert similar effects on activation of the TOP12 reporter mediated by the oncogenic form of ß-catenin (P. Hayward unpublished).
These results confirm and extend our observations in vivo and support the notion that the effects of Notch on Wnt signalling are mediated through a direct negative regulation of the activity of Armadillo. To test whether these effects are restricted to Drosophila Notch we have tested the ability of mouse Notch1 to modulate Wnt signalling in HEK-293T cells. A previous report has indicated that Notch1 NICD can suppress ß-catenin mediated Wnt signalling in Notch1 mutant keratinocytes (Nicolas et al., 2003). We have tested the ability of two different forms of membrane tethered Notch1 to modulate Wnt signalling (Fig.5E, F). One form delN – N1, a version of ΔE which removes all but 13 amino acids of the extracellular domain (Mumm et al., 2000)(see Fig 5A), can undergo spontaneous cleavage and activate a CBF reporter (Fig.5H). This form can also suppress ß-catenin activity. A second membrane tethered form LNR-N1 (see Fig.5A), a version of NLNR is rarely cleaved (Mumm et al., 2000) and only activates the CBF reporter very weakly (Fig.5H), but still strongly suppresses the activity of ß-catenin (Fig.5F).
These observations, together with the observation that Notch cannot inhibit Wnt reporter activity driven by a LEF1-VP16 fusion protein confirm and extend the results from Drosophila that indicate that Notch has an ability to interfere with the activity of ß-catenin. They also support the notion that this effect might not require the cleavage of Notch or its ability to activate transcription. The effects of Notch on the activity of ß-catenin contrast with those of a soluble form of Frizzled8 which, as shown previously are effective in suppressing Wnt induced Wnt signalling (Brennan et al., 2004) but are not able to suppress ß-catenin induced Wnt signalling (Fig.5G).
Notch can regulate Armadillo independently of Sgg/GSK3ß
The results described above show that Notch modulates the amount and the activity of Armadillo and that this effect is different from that mediated by NICD. To explore these relationships further we have analysed the effects of Notch loss of function on the stability of Armadillo.
In the imaginal discs, cells lacking shaggy function exhibit elevated levels of Armadillo that is enriched in the neighbourhood of the adherens junctions (Fig. 6A-C). In contrast, loss of Notch function does not alter the levels of endogenous Armadillo in a reproducible manner, although some times we have observed an increased accumulation of Armadillo in the neighbourhood of the DV boundary (see legend to Fig.6). However simultaneous loss of shaggy and Notch function results in small clones of cells in which Armadillo is not restricted to the adherens junctions as it is in shaggy mutants, but it is now distributed throughout the cytoplasm (Fig.6D-F).
Figure 6. Effects of loss of function of Notch and shaggy on the stability of Armadillo.
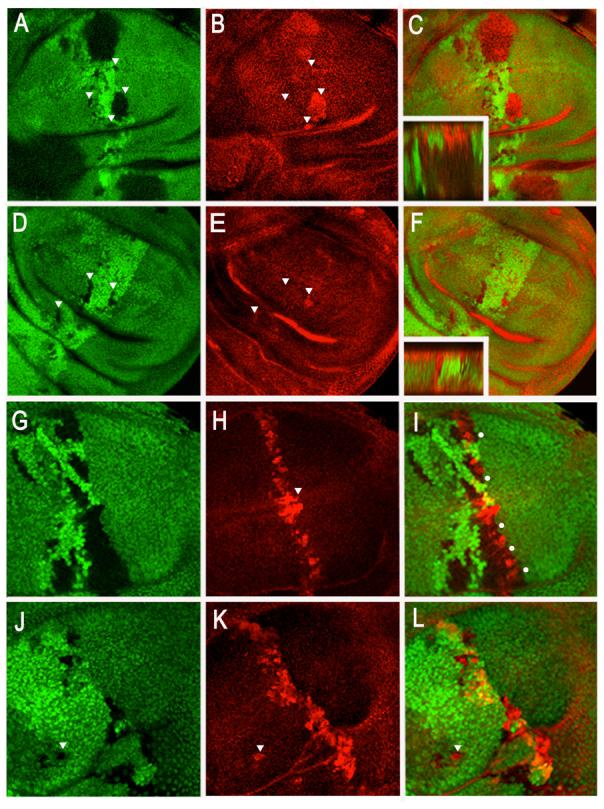
Clones of wild type and cells mutant for sgg, Notch or Notch and sgg were induced using the FLP recombinase system and were identified by the loss of GFP (see materials and methods). (A-C) Loss of function of shaggy results in a cell autonomous elevation of the levels of Armadillo, which remains largely associated with adherens junctions (inset in C in which apical is at the top). Notice also that only clones of a certain size (> about five cells) show the elevated levels of Armadillo; this is probably due to the long perdurance of Shaggy. The epithelium looks very thick (compare to inset in F) because the loss of sgg affects the epithelial organisation of the cells (Martinez Arias unpublished). (D-F) Simultaneous loss of Notch and shaggy results in very elevated levels of Armadillo that now appear delocalised in the cytoplasm of the cell (inset in F). The clones are small. Loss of function of Notch affects cell proliferation ((de Celis and Garcia-Bellido, 1994) and see also J-L) and this effect appears to be epistatic over the increased size of loss of function of sgg. (G-I) Expression of wild type Armadillo results in a Wingless dependent stabilisation of Armadillo (see also Figure 3 and S2). When wild type Armadillo is expressed under the control of ptcGAL4, high levels of Armadillo are observed in a narrow band at the AP border where the levels of ptcGAL4 expression are highest and, in particular, at the DV boundary (arrow in H) where the levels of Wingless are highest. Clones of wild type cells (loss of GFP) do not change the instability of the ectopic Armadillo (dots indicate regions in clones where Armadillo is not accumulated) . (J-L) Loss of function of Notch results in the stabilisation of the ectopic Armadillo. Notice that in this disc, which is heterozygous for Notch (Df(1)N81k/+) the dppGal4 driven expression of Armadillo is broader and contains more cells maintaining high levels of Armadillo than in wild type. Furthermore, within the clones of cells lacking Notch there are more cells with high levels of Armadillo than there are in wild type clones (compare with G-I).Notice that in some instances clones, that lie far away from that AP boundary (arrow head) maintain the expression of Armadillo. The clones are small because loss of function of Notch affects cell proliferation (see above). N.B. The effects of loss of function of Notch alone on the stability of endogenous Armadillo are not reproducible, In general we do not observe changes in the levels of Armadillo as a result of loss of Notch function (A. Martinez Arias unpublished), but in some experiments we observe an elevation in the levels of Armadillo. This elevation is always observed in the neighbourhood of the Dorsal Ventral boundary (A. Martinez Arias unpublished). Unfortunately this effect is not reproducible and therefore should remain anecdotal. It might reflect the existence of a very unstable pool of Armadillo that is not easy to fix and which is increased in the absence of sgg or by the ectopic expression of higher levels of wild type.
We were surprised to observe that removal of Notch function has no reproducible effects on the levels of Armadillo that can be detected in the presence of Shaggy. We reasoned that perhaps this is due to the fact that the hypophosphorylated form of Armadillo is in very small amounts due to efficiency of the Armadillo destruction machinery (Tolwinski and Wieschaus, 2001) and that this is a pool difficult to fix. Notch might act preferentially on this pool and therefore, in order to see the effects of Notch loss of function, the amounts of soluble Armadillo have to be above a certain level, as in the case of sgg mutant cells. To test this we saturated the levels of Armadillo by over-expressing high levels of full length Armadillo and then observed the effect of loss of Notch function on these saturating amounts (Fig.6G-L). Over-expression of Armadillo leads to its accumulation in a salt and pepper pattern which reveals a requirement for the cell cycle (Marygold and Vincent, 2003) and highlights its dependence on Wingless signalling (Fig.6G-I and Fig.S3). In the absence of Notch, the added Armadillo is consistently stabilised (Fig.6J-L) and this effect can be shown to be independent of Wingless (Fig.S3 and A. Martinez Arias unpublished.).
This observation mirrors the fact that gain of function of Notch eliminates any excess added Armadillo (Fig.4C, D) and indicates that Notch can regulate the stability of Armadillo and have effects on the equilibria of the different pools.
The relationships that we have observed between Notch and Armadillo, as well as between Notch and a form of Armadillo that is resistant to Shaggy mediated degradation, led us to enquire whether Notch could revert the effects of removal of Shaggy/GSK3. To do this we expressed TNotch in clones of cells which have lost shaggy in the developing wing disc. Loss of shaggy generates large clones with cell autonomous high levels of Wnt signalling as revealed by high levels of Armadillo and ectopic expression of targets of Wingless signalling e.g. senseless (Fig.7A-D). When TNotch is expressed in cells that have lost shaggy, Armadillo levels are returned to wild type and the transcriptional response is abolished (Fig.7E-H).
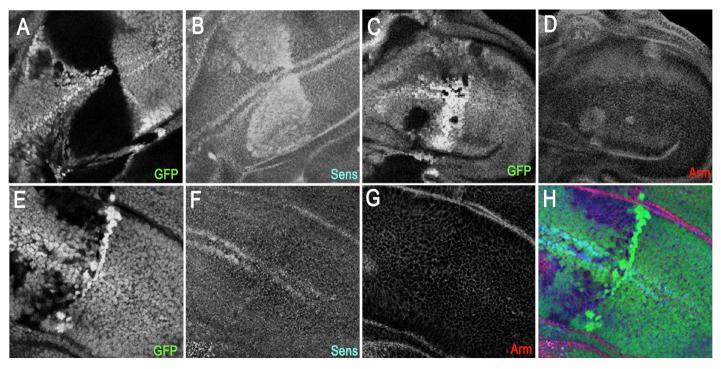
Figure 7. TNotch can suppress Wingless signalling induced by the loss of function of GSK3ß/Shaggy.
Wing pouch regions of third intar larval discs with clones of cells mutant for sgg marked by the absence of GFP (green) in a wild type background or in which TNotch is expressed in the clones. Anterior is to the left and posterior to the right. (A-D) Detail of a third larval instar wing disc harbouring clones of cells mutant for shaggy. (A, B) Loss of sgg function (black in A and C) leads to ectopic expression of the high threshold target of Wingless signalling Senseless (blue in B) and ectopic elevation of Armadillo (red in D). (E-H) Clones of cells lacking sgg which also express TNotch. TNotch reduces the ectopic expression of Senseless (F) and the elevation of Armadillo (H). The effects on senseless is fully penetrant but that on Armadillo can be variable (A. Martinez Arias unpublished).
These results support the observation that TNotch can regulate the activity of Armadillos10, which is resistant to Shaggy mediated regulation and indicate that the effects of Notch on Armadillo are independent of and acting on the Wingless pathway downstream of Shaggy/GSK3.
Armadillo associates with Notch in Drosophila
Our observations indicate a close functional association between Armadillo and Notch. One possibility is that the effects of Notch are indirect and are mediated by some proteins associated with a Su(H)-independent activity. Although this may well be the case, it is also possible that Armadillo is part of this complex. This possibility is suggested by the observation that Armadillo and Notch show a high degree of co-localisation at the adherens junction of the epidermal cells of the wing disc ((Fehon et al., 1991; Lamb et al., 1998) and A. Martinez Arias unpublished). To test if these observations reflect an association between Notch and Armadillo in the cell, we immunoprecipitated Notch from developing embryos and searched for Armadillo amongst the co-immunoprecipitated proteins. We tested two different anti-Notch antibodies and in both cases Armadillo protein was detected in the same protein complex as the immunoprecipitated Notch protein (Fig.8B). Interestingly, the predominant form of Notch protein detected in these assays is unprocessed and uncleaved (Kidd and Lieber, 2002), suggesting that this complex is membrane associated. The reverse experiment in which Armadillo protein is immunoprecipitated was also undertaken; here an unprocessed and uncleaved form of Notch was found to be associated with Armadillo (P. Hayward unpublished). Previous experiments have indicated that Dishevelled, another element of Wnt signalling, can associate with Notch in a yeast-two-hybrid assay. We have confirmed this and further shown this association in the same immunoprecipiates from embryos in which we find the complex between Notch and Armadillo (Fig.8B). Other proteins such as E-cadherin and nuclear lamin were not detected in the immunoprecipiates (P. Hayward unpublished). These results indicate that the intracellular domain of Notch and a proportion of the Armadillo protein of the cell are associated in the same protein complex. Preliminary data suggests that this association is preferentially mediated by the region C- terminal to the cdc10/ANK repeats (P. Sanders unpublished) and such an interaction might be an element in the functional interactions that we have described above.
Figure 8. Armadillo associates with Notch in Drosophila embryos.
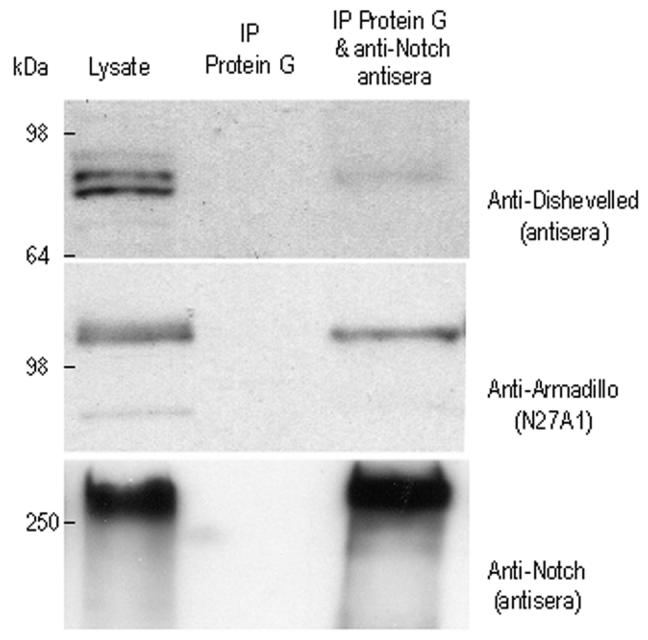
Notch protein was immunoprecipitated from wild type embryos and the presence of associated proteins was assessed by Western blot. The majority of Notch protein present in embryo lysate and immunoprecipitated was the full-length protein (>250 kDa). In Drosophila the predominant form of Notch at the cell surface is full length unlike in mammalian cells, where the majority of Notch molecules at the cell surface are furin cleaved heterodimeric molecule, this lack of furin cleavage does not seem to affect ability of the full length Notch molecule to signal in Drosophila (Kidd and Lieber, 2002). Note Notch protein (lower panel) was not detected in the Protein-G sepharose immunoprecipitation reaction. Detected in associated with immunoprecipitated Notch were both Armadillo (middle panel) and Dishevelled (upper panel) protein. (Axelrod et al., 1996; Zecchini et al., 1999). In these experiments the lysate lane represents 1/20th of the total protein added to the immunoprecipitation reaction. Typically we observe that the immunoprecipitated Notch reflects about 5-10% of the total Notch present in the lysate, associated with this Notch typically we observe between 0.5 –3% of the total Armadillo present and less than 0.5% of the total Dishevelled present. Given that the pool of Armadillo associated with Notch is not associated with E-cadherin it is only to be expected that this Notch associated fraction of Armadillo is very small protportion of the total cellular Armadillo.
DISCUSSION
Wnt signalling plays crucial and diverse roles in normal and pathological situations and therefore it is not surprising that the activity of its key effector, Armadillo/ß-catenin is tightly regulated (Giles et al., 2003; Polakis, 2000; Wodarz and Nusse, 1998). The precise mechanism whereby Wnt proteins elicit the activity of ß-catenin is still under scrutiny but it is generally agreed that the stability and amount of cytoplasmic Armadillo/ß-catenin are rate-limiting steps in the signalling event (Gottardi and Gumbiner, 2001; Lee et al., 2003). This pool of Armadillo/ß-catenin is under very tight control by a destruction complex assembled on Axin, which together with Shaggy/GSK3 are the main targets of Wnt signalling (Wodarz and Nusse, 1998). However there is increasing evidence that high levels of cytoplasmic Armadillo/ß-catenin are not sufficient to promote Wnt signalling (Brennan et al., 2004; Guger and Gumbiner, 2000; Lawrence et al., 2001; Staal et al., 2002). Recently emphasis has been placed on the observation that Axin can regulate the activity of Armadillo/ß-catenin in a Shaggy/GSK3 independent manner (Tolwinski et al., 2003; Tolwinski and Wieschaus, 2004b). This has led to the conclusion that Wnt regulates the activities of Shaggy/GSK3 and Axin co-ordinately and that there might be other factors contributing to the control of Armadillo/ß-catenin activity. Consistent with this possibility it has been reported that Wnt signalling can regulate the activity of stable oncogenic forms of ß-catenin (Suzuki et al., 2004)
Here we have shown that Notch signalling provides an important input into Wnt signalling in Drosophila by associating with Armadillo and regulating its levels and activity during Wingless signalling (Fig.9). This activity of Notch which is different and probably independent of that which mediates CBF1/Su(H) dependent signalling, lies functionally downstream of Shaggy/GSK3 and targets the concentration and activity of the hypophosphorylated form of Armadillo. It can also modulate the activity of an oncogenic form of vertebrate ß-catenin and we have demonstrated that this functional interaction between Notch and Armadillo extends to the vertebrate system, with mNotch1 regulating the activity of ß-catenin in tissue culture cells.
Figure 9. Modulation of Wnt signalling by Notch in Drosophila (see text for details of interactions).
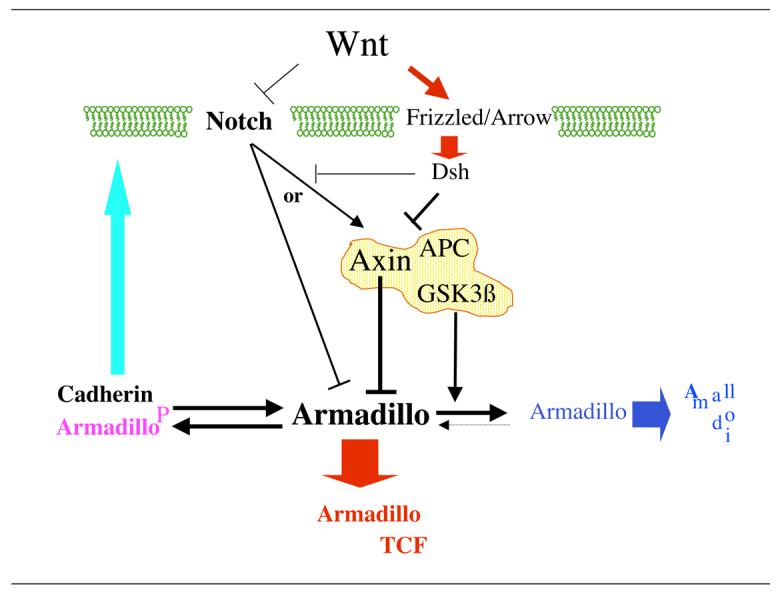
In the steady state, Armadillo exists in a number of molecularly distinct pools which appear to be in equilibrium. Armadillo associates readily with Cadherin and participated in the dynamics of adherens junctions. On the other hand, its association with a complex, which includes Axin and APC, leads to its phosphorylation on the N terminus by GSK3ß (Shaggy) and the delivery of the phosphorylated form to the proteasome where it is degraded. In addition to serving as a scaffold for GSK3ß mediated phosphorylation of Armadillo, Axin can prevent the formation of its active complex with TCF in a GSK3ß independent manner. Our results indicate that Notch modulates the activity and amounts of hypophosphorylated Armadillo either by targeting the GSK3ß independent activity of Axin or via an independent mechanism. Wnt signalling through a Frizzled/Arrow (LRP5/6) heterodimer activates Dishevelled which inactivates the complex, destroying Axin and inhibiting the N terminal phosphorylation of Arm. Wnt has been shown to bind to the extracellular domain of Notch (Brennan et al., 1999a; Wesley, 1999) and Dishevelled to the intracellular domain ((Axelrod et al., 1996)and this work) and this binding is likely to be responsible for down regulating the modulatory activity of Notch. The net effect of the inactivation of Notch and the Axin based complex results in an efficient accumulation of Armadillo in the nucleus and its interaction with TCF.
A role for Notch in the modulation of Wnt signalling has been inferred from genetic analysis. However, although these results indicate that Notch antagonises Wnt signalling, alone do not provide insights into the mechanism of the interaction. Our work does and it is likely that the molecular interactions that we report underpin the observed modulation of Wnt signalling by Notch (Martinez Arias, 2002). Wingless signalling can be activated in vivo in the absence of Notch and this activation does not require Dishevelled (Brennan et al., 1999a; Lawrence et al., 2001). Our observations that removal of Notch in cl8 cells leads to activation of a synthetic Wnt reporter confirm this and suggest a direct regulatory effect of Notch on the mechanism of Wnt signalling. Furthermore, the effects of Notch on the activated form of Armadillo offer an explanation for why removal of Notch can bypass a requirement for Dishevelled. It may well be that even under steady state conditions there is a small amount of hypophosphorylated, active Armadillo/ß-catenin which escapes the Axin/GSK3ß mediated degradation. Given the high specific activity of this molecule (Lee et al., 2001), it is not surprising that there might be further mechanisms that controls it. Notch appears to be an essential part of these mechanisms and in its absence this active form of Armadillo would operate even in the absence of Dishevelled. Axin is also likely to be involved in the regulation of the active form (Tolwinski et al., 2003) and we have observed that Axin can also suppress the effects of an activated form of Armadillo (A. Martinez Arias unpublished.). It will be of interest to explore the relationships between Notch and Axin.
The mechanism whereby Notch regulates Armadillo is not yet clear but our results suggest that it involves degradation of its active form. One possibility is that Notch is involved in the GSK3ß independent activity of Axin but, in addition, it may also play a role in the canonical degradation step since it has been observed that in addition to Dishevelled and Armadillo, the intracellular domain of Notch interacts with GSK3ß (Espinosa et al., 2003; Foltz et al., 2002) and there are reports of genetic interactions with sgg, the gene encoding Drosophila GSK3ß ((Brennan et al., 1999b; Ruel et al., 1993)and also here).
Previous studies have implicated Deltex and Dishevelled as important elements of the interaction between Notch and Wingless signalling (Axelrod et al., 1996; Martinez Arias et al., 2002; Ramain et al., 2001). Both proteins bind Notch, but they do so in different places. Deltex binds to the cdc10/ANK repeats (Matsuno et al., 1995) and promotes Su(H)-independent Notch signalling. On the other hand, Dishevelled binds within a broad region C-terminal to this domain and reduces the Su(H)-independent activity of Notch ((Axelrod et al., 1996; Ramain et al., 2001) and see also (Zecchini et al., 1999)). Here we have shown that Armadillo also interacts with Notch and it probably does so through the same broad region that binds Dishevelled. It is likely that other proteins that participate in this process also bind here. Mutations in Notch that impair this domain result in Notch receptors that interfere with Wnt signalling (Ramain et al., 2001) and we have observed that its deletion reduces the efficiency with which the intracellular domain of Notch to affects the levels and activity of Armadillo (P. Hayward unpublished). Together these observations underscore the role of this region of Notch in mediating interactions between Notch and Wnt signalling by targeting the active form of Armadillo/ß-catenin.
As we have demonstrated here, the relationship between Notch and Armadillo in Drosophila extends to their vertebrate homologues, Notch1 and ß-catenin. This interaction, rather than an interaction of Dishevelled with Notch/CBF signalling, might reflect the functional relationships between the two signalling systems that have been reported during the development of the skin (Lowell et al., 2000; Nicolas et al., 2003; Zhu and Watt, 1999), the immune system (Radtke et al., 1999; Reya et al., 2000) and somitogenesis (Aulehla et al., 2003; Dale et al., 2003; Pourquie, 2003). In these instances Wnt and Notch drive alternate fates (skin and immune system) or act antagonistically (somites) perhaps by a combination of their individual pathways and the modulatory interaction that we have described here. One consequence of this modulatory interaction might also be the observed tumour suppressor function of Notch-1 in the mouse skin where removal of Notch-1 results in the generation of tumours associated with an increase in the levels of active ß-catenin and Wnt signalling (Nicolas et al., 2003). Whilst some of the elevation of ß-catenin in these cells might be a secondary consequence of activation of Wnt signalling, our observations suggests that the loss of Notch-1 can also contribute to this increase by allowing the activation of ß-catenin. In a different study carboxyl-terminal deletions in Notch-1, which include the region that binds Dishevelled and Armadillo, enhance the oncogenic effects of a chimeric E2A-PBX1 protein (Feldman et al., 2000). It is possible that some of this effect is due to misregulation of ß-catenin in the tumours.
In summary, we have shown that Notch provides a modulatory input in the activity of Armadillo/ß-catenin (Fig.9). This modulation provides two functions: it establishes a threshold for Wnt signalling that is likely to play an important role in the patterning of tissues and the assignation of cell fates during development (Martinez Arias, 2002) and, in addition it provides a stringent regulation for the activated form of Armadillo/ß-catenin. The second function might be very crucial in pathological situations and might contribute to the understanding of Notch as a tumour suppressor (Radtke and Raj, 2003).
Supplementary Material
Acknowledgements
We want to thank H. Bellen for the anti-Senseless antibody, R. Nusse for the anti Dishevelled antibody, H. Bellen for anti-senseless antibody, I. Duncan for anti-Distalless antibody and the Developmental Studies Hybridoma Bank for N2 7A1, C17.9C6 and E7 monoclonal antibodies. A. Muller for a generous supply of anti-Armadillo antibody and discussions on the biochemistry of Armadillo. T. Klein for fly stocks. K. Beckett, N. Gorfinkiel andV. Morel for discussions and comments. This work is supported by The Wellcome Trust (AMA, KB, PH, TB), the Medical Research Council (PS), the Breast Cancer Research program, US Department of Defense (RG) and the Howard Hughes Medical Institute (NP).
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271:1826–32. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- Barolo S, Stone T, Bang AG, Posakony JW. Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 2002;16:1964–76. doi: 10.1101/gad.987402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, Haas SA, Consortium HF, Paro R, Perrimon N. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science. 2004;303:832–5. doi: 10.1126/science.1091266. [DOI] [PubMed] [Google Scholar]
- Brennan K, Gonzalez-Sancho JM, Castelo-Soccio LA, Howe LR, Brown AM. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins. Oncogene. 2004;23:4873–84. doi: 10.1038/sj.onc.1207642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan K, Klein T, Wilder E, Martinez Arias A. Wingless modulates the effects of dominant negative notch molecules in the developing wing of Drosophila. Dev Biol. 1999a;216:210–29. doi: 10.1006/dbio.1999.9502. [DOI] [PubMed] [Google Scholar]
- Brennan K, Tateson R, Lieber T, Couso JP, Zecchini V, Matinez Arias A. The abruptex mutations of notch disrupt the establishment of proneural clusters in Drosophila. Dev Biol. 1999b;216:230–42. doi: 10.1006/dbio.1999.9501. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–9. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Cox RT, Pai LM, Miller JR, Orsulic S, Stein J, McCormick CA, Audeh Y, Wang W, Moon RT, Peifer M. Membrane-tethered Drosophila Armadillo cannot transduce Wingless signal on its own. Development. 1999;126:1327–35. doi: 10.1242/dev.126.6.1327. [DOI] [PubMed] [Google Scholar]
- Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421:275–8. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Garcia-Bellido A. Roles of the Notch gene in Drosophila wing morphogenesis. Mech Dev. 1994;46:109–22. doi: 10.1016/0925-4773(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen SM. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–25. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- Endo Y, Osumi N, Wakamatsu Y. Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development. 2002;129:863–73. doi: 10.1242/dev.129.4.863. [DOI] [PubMed] [Google Scholar]
- Endo Y, Osumi N, Wakamatsu Y. Deltex/Dtx mediates NOTCH signaling in regulation of Bmp4 expression in cranial neural crest formation during avian development. Dev Growth Differ. 2003;45:241–8. doi: 10.1046/j.1524-4725.2003.693.x. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–35. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Johansen K, Rebay I, Artavanis-Tsakonas S. Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J Cell Biol. 1991;113:657–69. doi: 10.1083/jcb.113.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Hampton T, Cleary ML. A carboxy-terminal deletion mutant of Notch1 accelerates lymphoid oncogenesis in E2A-PBX1 transgenic mice. Blood. 2000;96:1906–13. [PubMed] [Google Scholar]
- Foltz DR, Santiago MC, Berechid BE, Nye JS. Glycogen synthase kinase-3beta modulates notch signaling and stability. Curr Biol. 2002;12:1006–11. doi: 10.1016/s0960-9822(02)00888-6. [DOI] [PubMed] [Google Scholar]
- Furriols M, Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol. 2001;11:60–4. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol. 2001;11:R792–4. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM. A mode of regulation of beta-catenin signaling activity in Xenopus embryos independent of its levels. Dev Biol. 2000;223:441–8. doi: 10.1006/dbio.2000.9770. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual: Cold Spring Harbor Laboratory. 1988 [Google Scholar]
- Kidd S, Lieber T. Furin cleavage is not a requirement for Drosophila Notch function. Mech Dev. 2002;115:41–51. doi: 10.1016/s0925-4773(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Kidd S, Lieber T, Young MW. Ligand-induced cleavage and regulation of nuclear entry of Notch in Drosophila melanogaster embryos. Genes Dev. 1998;12:3728–40. doi: 10.1101/gad.12.23.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Martinez Arias A. Different spatial and temporal interactions between Notch, wingless, and vestigial specify proximal and distal pattern elements of the wing in Drosophila. Dev Biol. 1998;194:196–212. doi: 10.1006/dbio.1997.8829. [DOI] [PubMed] [Google Scholar]
- Klein T, Martinez Arias A. The vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Development. 1999;126:913–25. doi: 10.1242/dev.126.5.913. [DOI] [PubMed] [Google Scholar]
- Klein T, Seugnet L, Haenlin M, Martinez Arias A. Two different activities of Suppressor of Hairless during wing development in Drosophila. Development. 2000;127:3553–66. doi: 10.1242/dev.127.16.3553. [DOI] [PubMed] [Google Scholar]
- Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–7. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–96. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–29. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Ward RE, Schweizer L, Fehon RG. Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Mol Biol Cell. 1998;9:3505–19. doi: 10.1091/mbc.9.12.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N, Klein T, Brennan K, Martinez Arias A. Structural requirements for notch signalling with delta and serrate during the development and patterning of the wing disc of Drosophila. Development. 2000;127:3185–95. doi: 10.1242/dev.127.14.3185. [DOI] [PubMed] [Google Scholar]
- Lawrence N, Langdon T, Brennan K, Martinez Arias A. Notch signaling targets the Wingless responsiveness of a Ubx visceral mesoderm enhancer in Drosophila. Curr Biol. 2001;11:375–85. doi: 10.1016/s0960-9822(01)00120-8. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol. 2001;154:983–93. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The Roles of APC and Axin Derived from Experimental and Theoretical Analysis of the Wnt Pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993;7:1949–65. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A. New alleles of Notch draw a blueprint for multifunctionality. Trends Genet. 2002;18:168–70. doi: 10.1016/s0168-9525(01)02635-x. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A. Wnts as morphogens? The view from the wing of Drosophila. Nat Rev Mol Cell Biol. 2003;4:321–5. doi: 10.1038/nrm1078. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A, Zecchini V, Brennan K. CSL-independent Notch signalling: a checkpoint in cell fate decisions during development? Curr Opin Genet Dev. 2002;12:524. doi: 10.1016/s0959-437x(02)00336-2. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Vincent JP. Armadillo levels are reduced during mitosis in Drosophila. Mech Dev. 2003;120:157–65. doi: 10.1016/s0925-4773(02)00439-2. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995;121:2633–44. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Nagao M, Ebert BL, Ratcliffe PJ, Pugh CW. Drosophila melanogaster SL2 cells contain a hypoxically inducible DNA binding complex which recognises mammalian HIF-binding sites. FEBS Lett. 1996;387:161–6. doi: 10.1016/0014-5793(96)00484-x. [DOI] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–21. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- Nye JS, Kopan R, Axel R. An activated Notch suppresses neurogenesis and myogenesis but not gliogenesis in mammalian cells. Development. 1994;120:2421–30. doi: 10.1242/dev.120.9.2421. [DOI] [PubMed] [Google Scholar]
- Pai LM, Orsulic S, Bejsovec A, Peifer M. Negative regulation of Armadillo, a Wingless effector in Drosophila. Development. 1997;124:2255–66. doi: 10.1242/dev.124.11.2255. [DOI] [PubMed] [Google Scholar]
- Peel DJ, Johnson SA, Milner MJ. The ultrastructure of imaginal disc cells in primary cultures and during cell aggregation in continuous cell lines. Tissue Cell. 1990;22:749–58. doi: 10.1016/0040-8166(90)90069-l. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994a;166:543–56. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994b;120:369–80. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- Pourquie O. The segmentation clock: converting embryonic time into spatial pattern. Science. 2003;301:328–30. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–67. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Ramain P, Khechumian K, Seugnet L, Arbogast N, Ackermann C, Heitzler P. Novel Notch alleles reveal a Deltex-dependent pathway repressing neural fate. Curr Biol. 2001;11:1729–38. doi: 10.1016/s0960-9822(01)00562-0. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fehon RG, Artavanis-Tsakonas S. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993;74:319–29. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell. 1991;67:687–99. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- Reya T, O'Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000;13:15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993;362:557–60. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS. wingless refines its own expression domain on the Drosophila wing margin. Nature. 1996;384:72–4. doi: 10.1038/384072a0. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–38. [PubMed] [Google Scholar]
- Schweisguth F, Lecourtois M. The activity of Drosophila Hairless is required in pupae but not in embryos to inhibit Notch signal transduction. Dev Genes Evol. 1998;208:19–27. doi: 10.1007/s004270050149. [DOI] [PubMed] [Google Scholar]
- Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–98. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–58. [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–67. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–8. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–60. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–36. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–45. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Dong Chen W, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–18. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development. 2001;128:2107–17. doi: 10.1242/dev.128.11.2107. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. A Nuclear Function for Armadillo/beta-Catenin. PLoS Biol. 2004a;2:E95. doi: 10.1371/journal.pbio.0020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. Rethinking WNT signaling. Trends Genet. 2004b;20:177–81. doi: 10.1016/j.tig.2004.02.003. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Samos CH, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature. 1994;368:342–4. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- Wesley CS. Notch and wingless regulate expression of cuticle patterning genes. Mol Cell Biol. 1999;19:5743–58. doi: 10.1128/mcb.19.8.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, Lee JS, Ishimoto A. Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. J Biol Chem. 1998;273:32353–9. doi: 10.1074/jbc.273.48.32353. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–44. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- Zecchini V, Brennan K, Martinez-Arias A. An activity of Notch regulates JNK signalling and affects dorsal closure in Drosophila. Curr Biol. 1999;9:460–9. doi: 10.1016/s0960-9822(99)80211-5. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–98. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.