Abstract
The p38α/β mitogen-activated protein kinase (MAPK) pathway promotes skeletal myogenesis, but the mechanisms by which it is activated during this process are unclear. During myoblast differentiation, the promyogenic cell surface receptor Cdo binds to the p38α/β pathway scaffold protein JLP and, via JLP, p38α/β itself. We report that Cdo also interacts with Bnip-2, a protein that binds the small guanosine triphosphatase (GTPase) Cdc42 and a negative regulator of Cdc42, Cdc42 GTPase-activating protein (GAP). Moreover, Bnip-2 and JLP are brought together through mutual interaction with Cdo. Gain- and loss-of-function experiments with myoblasts indicate that the Cdo–Bnip-2 interaction stimulates Cdc42 activity, which in turn promotes p38α/β activity and cell differentiation. These results reveal a previously unknown linkage between a cell surface receptor and downstream modulation of Cdc42 activity. Furthermore, interaction with multiple scaffold-type proteins is a distinctive mode of cell surface receptor signaling and provides one mechanism for specificity of p38α/β activation during cell differentiation.
Introduction
Differentiation of vertebrate skeletal myoblasts into multinucleated myofibers is a multistage process that involves the coordinated activation of a cell type–specific transcriptional program and morphological changes that include elongation, alignment, and cell–cell fusion (Pownall et al., 2002; Tapscott, 2005). Members of the myogenic bHLH family (MyoD, Myf5, myogenin, and MRF4) are lineage-specific transcription factors that direct the differentiation process in conjunction with nonskeletal muscle–specific transcription factors such as Mef2 (Pownall et al., 2002; Tapscott, 2005). The activity of such factors is under tight posttranslational control by signal transduction pathways, including the p38α/β MAPK pathway (Lluis et al., 2006). p38α/β is activated during myogenic differentiation in vitro, and differentiation is blocked by chemical inhibitors of p38α/β (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000). p38α-null myoblasts are deficient in cell cycle arrest, expression of muscle-specific proteins, and myotube formation, and mice lacking p38α display delayed myofiber growth and maturation (Perdiguero et al., 2007). p38α/β directly phosphorylates several proteins that regulate myogenesis, including Mef2 isoforms, the myogenic bHLH heterodimeric partner E47, the SWI–SNF chromatin remodeling complex subunit BAF60, and the RNA decay-promoting factor KSRP (Wu et al., 2000; Simone et al., 2004; Briata et al., 2005; Lluis et al., 2005).
RD rhabdomyosarcoma cells (cancer cells of the muscle lineage that have low differentiation capability) are deficient in p38α/β activity in response to differentiation-inducing culture conditions, and enforced p38α/β activation in these cells by expression of activated MKK6 (an immediate upstream activating kinase for p38) rescued myogenesis (Puri et al., 2000). Cytokines and various types of cellular stress are activators of the p38α/β and other pathways (Zarubin and Han, 2005); however, treatment of RD cells with TNFα, sorbitol or UV light failed to rescue differentiation despite activation of p38α/β, revealing that differentiation- and stress-induced programs are distinct (Puri et al., 2000). Furthermore, the p38α/β pathway functions as a switch in muscle satellite cells, required initially to phosphorylate unidentified substrates that activate cell cycle entry and subsequently targeting substrates named in the previous paragraph to promote cell differentiation (Jones et al., 2005). Presumably, these distinct roles of p38α/β signaling require appropriate concentrations of p38α/β to become activated at specific times and subcellular locations. However, the spatiotemporal regulatory mechanisms by which reiteratively used signaling pathways (like p38α/β) achieve such specificity are, in most cases, unclear.
The Rho family of small GTPases regulates many biological processes, including cytoskeletal dynamics, cell polarity, signal transduction, and transcription (Van Aelst and D'Souza-Schorey, 1997; Jaffe and Hall, 2005). They are therefore well positioned to coordinate the changes in both gene expression and cell morphology that characterize cell differentiation. The role in vertebrate myogenesis of one such GTPase, Cdc42, is controversial. The concentration of active GTP-bound Cdc42 is relatively low in proliferating myoblasts and increases severalfold in differentiating cells (Travaglione et al., 2005). Studies in which constitutively active and dominant-negative mutants of Cdc42 were expressed in different myoblast cell systems have produced contradictory results but, in general, both mutants interfered with myogenesis (Takano et al., 1998; Gallo et al., 1999; Meriane et al., 2000), suggesting that major perturbations in Cdc42 activity are not tolerated.
Like other small GTPases, Cdc42 cycles between an inactive GDP-bound state and an active GTP-bound state. The activity cycle is directly regulated by its interaction with stimulatory guanine nucleotide exchange factors and inhibitory GTPase-activating proteins (GAPs) and by interaction with additional proteins (Van Aelst and D'Souza-Schorey, 1997; Jaffe and Hall, 2005). A candidate regulator of Cdc42 is Bnip-2 (Low et al., 1999, 2000a,b). Bnip-2 is a 314-aa protein that harbors a single recognizable motif, a BCH domain that spans its C-terminal half. Cdc42GAP (also known as p50RhoGAP/ARHGAP1) contains a BCH domain in its noncatalytic N-terminal region, and Bnip-2 and Cdc42GAP interact via their respective BCH domains (Low et al., 1999, 2000b). The Bnip-2 BCH domain also binds Cdc42 itself (Low et al., 2000a). Transient expression of Bnip-2 in several cell types induces cellular elongation and membrane protrusions in a manner dependent on its ability to bind Cdc42 and on cellular Cdc42 activity (Zhou et al., 2005). That Bnip-2 binds Cdc42GAP, a negative regulator of Cdc42, yet has the ability to induce morphological alterations that require Cdc42 binding and Cdc42 activity, suggests that Bnip-2 might function as a scaffold for dynamic regulation of Cdc42 signaling. However, modulation of cellular Cdc42 activity by Bnip-2 has not been demonstrated.
Cdo is a cell surface receptor of the Ig superfamily that promotes myogenesis in vivo and in vitro (Kang et al., 1998, 2003; Cole et al., 2004). Primary myoblasts from Cdo−/− mice and C2C12 myoblasts that express Cdo siRNA differentiate defectively in culture, producing reduced levels of muscle-specific proteins and fusing into myotubes inefficiently (Cole et al., 2004; Takaesu et al., 2006). During myogenesis, the Cdo intracellular region binds to JLP, a scaffold protein for the p38α/β MAPK pathway and, via JLP, p38α/β (Takaesu et al., 2006). Cdo−/− myoblasts are deficient in differentiation-associated p38α/β activity, and their differentiation defect is specifically rescued by expression of activated MKK6 (Takaesu et al., 2006); however, how association of JLP/p38α/β with Cdo leads to activation of p38α/β is unknown. We report in this paper that the Cdo intracellular region binds Bnip-2 and that this interaction regulates Cdc42 activity, thus identifying a novel linkage between a cell surface receptor and regulation of Cdc42. Bnip-2 and JLP do not directly associate but are brought together through mutual interaction with Cdo, and Bnip-2 and Cdc42 stimulate p38α/β activation and myogenic differentiation. Interaction with multiple scaffold-type proteins is an unusual mode of cell surface receptor signaling and provides a mechanism by which p38α/β can be specifically activated to promote cell differentiation.
Results
Cdo interacts with Bnip-2
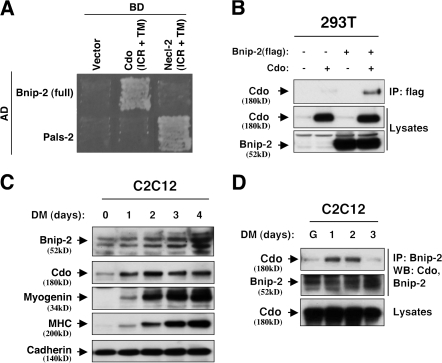
A yeast two-hybrid screen that used the Cdo intracellular region as bait previously identified JLP as a Cdo-interacting protein (Takaesu et al., 2006). This screen also identified positive clones encoding Bnip-2 (Fig. 1 A). The intracellular region of another Ig protein, Necl-2, did not interact as efficiently with Bnip-2, nor did Cdo interact as efficiently with a Necl-2 binding protein, Pals-2 (Shingai et al., 2003; Takaesu et al., 2006). Consistent with their interaction in yeast, Cdo and flag epitope–tagged Bnip-2 coimmunoprecipitated when transiently expressed in 293T cells (Fig. 1 A). Like Cdo, Bnip-2 is expressed endogenously in C2C12 myoblasts, and Bnip-2 levels increased during a 4-d time course of differentiation (Fig. 1 C). On SDS-PAGE of C2C12 cell lysates, Bnip-2 migrated as a series of bands (Fig. 1, C and D) that likely represent alternatively spliced variously phosphorylated isoforms (Low et al., 1999; unpublished data). To assess whether endogenous Cdo and Bnip-2 interact, C2C12 cells were harvested while proliferating in growth medium (GM) or over a 3-d time course after transfer to differentiation medium (DM). Cell lysates were immunoprecipitated with Bnip-2 antibodies and probed with Cdo antibodies (Fig. 1 D). Cdo coprecipitated with Bnip-2, with maximal interaction occurring over the first 2 d in DM, which is similar to Cdo's interaction with JLP (Takaesu et al., 2006).
Figure 1.
Cdo interacts with Bnip-2. (A) Yeast transformed with the indicated vectors for Gal4 DNA binding domain (BD) fused to the transmembrane (TM) plus intracellular regions (ICR) of Cdo or Necl-2 and Gal4 activation domain (AD) fused to Bnip-2 or Pals2 were plated on two-hybrid interaction-dependent selective medium. (B) Lysates of 293T cells transiently transfected with flag-tagged Bnip-2, Cdo, or control (−) expression vectors as indicated were immunoprecipitated (IP) with flag antibodies and then Western blotted with flag or Cdo antibodies. (C) Lysates of C2C12 cells cultured in GM to ∼80% confluence and then transferred to DM for the indicated times were subjected to Western blot analysis with the indicated antibodies. (D) Lysates of C2C12 cells that were proliferating in GM (G) or transferred to DM for the indicated times were immunoprecipitated with Bnip-2 antibodies and then Western blotted with Cdo or Bnip-2 antibodies.
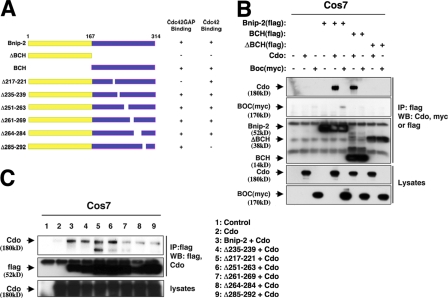
To identify the Cdo binding site in Bnip-2, a series of previously characterized flag-tagged Bnip-2 deletion mutants (Fig. 2 A; Low et al., 2000b; Zhou et al., 2005) were tested for their ability to coimmunoprecipitate Cdo in transiently transfected COS7 cells (Fig. 2 B). A construct comprising only the Bnip-2 BCH domain (aa 167–314) was as effective as full-length Bnip-2 in coprecipitating Cdo, but a construct lacking this domain failed to do so. Therefore, Bnip-2 binds to Cdo via its BCH domain. Boc is a protein that forms cis complexes with Cdo. Their respective ectodomains have strong sequence similarity but their intracellular regions are unrelated (Kang et al., 2002). Boc also coimmunoprecipitated with Bnip-2, but the interaction appeared less efficient and was only observed with full-length Bnip-2 (Fig. 2 B). A series of Bnip-2 mutants with small deletions in the BCH domain, which disrupt specific protein–protein interactions (Fig. 2 A; Low et al., 2000b; Zhou et al., 2005), was tested in an analogous manner. The deletion constructs Δ261–269, Δ264–284, and Δ285–292 each strongly diminished, but did not fully prevent, Cdo binding (Fig. 2 C). In contrast, Δ217–221, Δ235–239, and Δ251–263 bound Cdo normally, suggesting that a 31-aa region (261–292) near the C terminus of Bnip-2 encompasses the site of Cdo binding.
Figure 2.
Cdo binds the BCH domain of Bnip-2. (A) Schematic of Bnip-2 and Bnip-2 deletion mutants and their ability to bind Cdc42GAP and Cdc42 (summarized from Low et al., 2000b; Zhou et al., 2005). Note that Δ217–221 and Δ235–239 are also known as ΔM and ΔR, respectively (Low et al., 2000b; Zhou et al., 2005). (B) Lysates of COS7 cells transiently transfected with flag-tagged Bnip-2, flag-tagged BCH, flag-tagged ΔBCH, Cdo, myc-tagged Boc, or control (−) expression vectors as indicated were immunoprecipitated (IP) with flag epitope antibodies and then Western blotted with flag epitope, Cdo, or myc epitope antibodies. (C) Lysates of COS7 cells transiently transfected with flag-tagged Bnip-2, flag-tagged Bnip-2 deletion mutants, Cdo, or control (−) expression vectors as indicated were immunoprecipitated (IP) with flag epitope antibodies and then Western blotted with flag epitope or Cdo antibodies. For unknown reasons, a faster migrating Cdo degradation band is reproducibly seen when Cdo is coexpressed with the Δ217–221 mutant. Lower levels of this band are also seen when Cdo is coexpressed with the Δ251–263 and Δ261–269 mutants.
Bnip-2 positively regulates myogenic differentiation
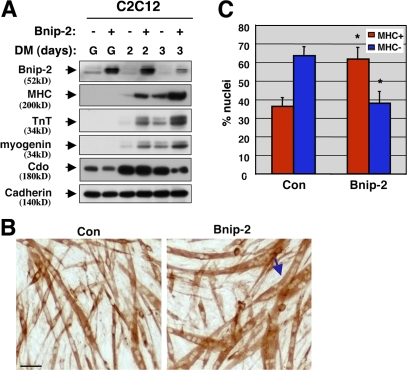
To explore a role for Bnip-2 in myogenesis, C2C12 cells with enhanced or diminished Bnip-2 levels were constructed by stable expression of a Bnip-2 cDNA or Bnip-2 siRNAs, respectively. Forced expression of a Bnip-2 cDNA generally increased overall immunoreactivity of Bnip-2 bands about fourfold (Fig. 3 A). Overexpression of Bnip-2 did not significantly alter the morphology or proliferation of C2C12 cells cultured in GM (unpublished data). However, when triggered to differentiate, cells that overexpressed Bnip-2 (C2C12/Bnip-2 cells) displayed a greater fraction of cell nuclei in myosin heavy chain–positive (MHC+) myotubes than did vector control cells (Fig. 3, B and C). Furthermore, some of the myotubes formed by C2C12/Bnip-2 cells had much larger numbers of nuclei than were observed with the vector control cells (Fig. 3 C, arrow). When analyzed for DM-induced expression of muscle-specific proteins, C2C12/Bnip-2 cells showed accelerated and enhanced levels of the differentiation markers myogenin, MHC, and troponin T relative to the control cultures (Fig. 3 A).
Figure 3.
Overexpression of Bnip-2 enhances myogenic differentiation. (A) Lysates of C2C12 cells stably transfected with a control expression vector lacking a cDNA (−) or with an expression vector harboring a Bnip-2 cDNA (+) were Western blotted with antibodies to Bnip-2, the indicated muscle-specific proteins, or Cdo and, as a control, with pan-cadherin antibody. (B) Photomicrographs of C2C12/Bnip-2 and vector control cells that were cultured in DM, fixed, and stained with an antibody to MHC. The arrow indicates a C2C12/Bnip-2 cell myotube with many more nuclei than are seen with the control cells under these conditions. Bar, 0.1 mm. (C) Quantification of myotube formation. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Asterisks indicate difference from control, P < 0.01. A level of myotube formation by control cells (∼35% nuclei in MHC+ cells) was selected so as to permit visualization of enhanced differentiation by Bnip-2.
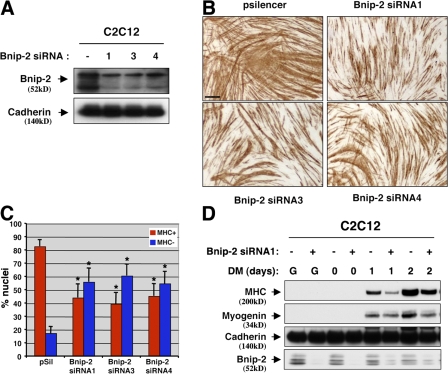
To assess the effects of diminished Bnip-2 levels on differentiation, three independent Bnip-2 sequences placed into the pSilencer siRNA vector were individually and stably expressed in C2C12 cells. Each sequence substantially reduced Bnip-2 protein levels (Fig. 4 A). Note that the most slowly migrating band was more variably affected than the faster migrating forms in Fig. 4 A and in later figures. This could represent a more stable species of Bnip-2 that is relatively resistant to RNAi-mediated knockdown or revelation of a protein nonspecifically recognized by the Bnip-2 antibody that comigrates with the top Bnip-2 band. C2C12 cells that expressed each siRNA sequence displayed a smaller percentage of cell nuclei in MHC+ myotubes, and the myotubes that formed were shorter and thinner, than control cells that expressed an irrelevant siRNA sequence (Fig. 4, B and C). Expression of MHC was delayed and reduced in Bnip-2 siRNA-expressing cells, whereas expression of myogenin was not substantially altered, perhaps because of incomplete knockdown of Bnip-2 (Fig. 4 D). These latter results suggest that myotube formation may be more sensitive to partial loss of Bnip-2 than is expression of muscle-specific proteins.
Figure 4.
RNAi-mediated depletion of Bnip-2 reduces myogenic differentiation. (A) Lysates of C2C12 cells stably transfected with pSilencer containing one of three independent Bnip-2 siRNA sequences (designated 1, 3, and 4) or pSilencer containing an irrelevant sequence (−) were Western blotted with Bnip-2 or, as a control, pan-cadherin antibodies. (B) Photomicrographs of C2C12 cells that express Bnip-2 siRNA sequences or an irrelevant sequence (pSilencer) as indicated, cultured in DM, fixed, and stained with an antibody to MHC. Bar, 0.5 mm. (C) Quantification of myotube formation. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Asterisks indicate difference from pSilencer control, P < 0.01. A level of myotube formation by control cells (∼80% nuclei in MHC+ cells) was selected so as to permit visualization of diminished differentiation by Bnip-2 siRNA. (D) Western blot analysis of C2C12 cells that express Bnip-2 siRNA sequences (+) or an irrelevant sequence (−) cultured in GM (G) or in DM for the indicated times.
Bnip-2 binds to Cdc42, Cdc42GAP, and, as is shown here, Cdo. The Bnip-2 deletion mutant Δ217–221 is selectively defective in Cdc42GAP binding. The Δ285–292 mutant does not bind to Cdc42 and is inefficient at binding Cdo but binds Cdc42GAP. The Δ264–284 mutant is inefficient at binding Cdo but interacts normally with Cdc42 and Cdc42GAP (Fig. 2, A and C; Low et al., 2000b; Zhou et al., 2005). The ability of these mutants to promote myotube formation was examined in a transient myogenesis assay that scores expression of MHC and myotube formation by transfectants (Kang et al., 2004; Takaesu et al., 2006). C2C12 cells were cotransfected with control or Bnip-2 expression vectors plus, to mark transfectants, a vector that drives expression of nuclear-localized β-galactosidase (β-gal). Transfection efficiencies of ∼10% were used to minimize fusion of independent β-gal+ transfectants. 48 h after transfection, the cultures were transferred to DM, and 72 h later the cells were double-stained for β-gal activity and for MHC expression. Note that when transfectants fused with nontransfected cells, many (often most) of the nuclei in the myotube became positive for β-gal activity because the cytoplasmically translated protein diffuses within the myotube (Kang et al., 2004; Takaesu et al., 2006). Expression of wild-type Bnip-2 stimulated MHC expression and production of multinucleated myotubes by β-gal+ transfectants (Fig. 5, A and B). The Δ217–221 mutant functioned similarly to wild-type Bnip-2, indicating that interaction with Cdc42GAP is not required for Bnip-2's promyogenic activity. In contrast, the Δ264–284 and Δ285–292 mutants lost the ability to promote myogenesis, suggesting that interaction with Cdc42 and/or Cdo are required for this activity (Fig. 5, A and B).
Figure 5.
Bnip-2 deletion mutants defective in binding Cdc42 and Cdo do not promote myogenic differentiation. (A) C2C12 cells were cotransfected with control, Bnip-2 or Bnip-2 deletion mutant expression vectors, and pQ-lacZ (a vector that drives expression of nuclear-localized β-gal to mark transfectants). Differentiated cultures were double stained for β-gal activity (blue) and for MHC expression (brown). Bar, 0.1 mm. (B) Quantification of C2C12 cell differentiation shown in A. Cultures were scored as MHC− or MHC+, with MHC+ cells further scored as having a single (1) nucleus, two to four nuclei, or greater than or equal to five nuclei. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results.
Cdo and Bnip-2 promote Cdc42 activity, and Cdc42 promotes myogenesis
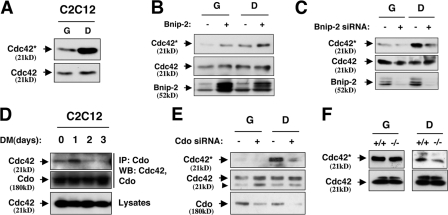
The concentration of GTP-bound (active) Cdc42 rises, and is maintained, when C2C12 cells are transferred into DM (Fig. 6 A; Travaglione et al., 2005). We therefore tested whether modulation of Bnip-2 levels altered the amount of active Cdc42 in these cells. C2C12/Bnip-2 cells displayed elevated levels of GTP-bound Cdc42 relative to vector control cells in both GM and DM (Fig. 6 B), whereas stable expression of Bnip-2 siRNA largely prevented the DM-induced increase in GTP-bound Cdc42 (Fig. 6 C). The concentration of active Cdc42 therefore correlates with Bnip-2 protein levels.
Figure 6.
Bnip-2 and Cdo regulate Cdc42 activity. (A) GTP-bound Cdc42 (Cdc42*) in C2C12 cells proliferating in GM (G) or cultured for 48 h in DM (D) was pulled down from cell lysates with GST-PAK-1 PBD beads and Western blotted with Cdc42 antibodies (top). Total levels of Cdc42 were determined by blotting straight lysates with Cdc42 antibodies (bottom). (B) Levels of GTP-bound and total Cdc42 in C2C12/Bnip-2 (+) or vector control (−) cells in GM or DM were assessed as described in A. (C) Levels of GTP-bound and total Cdc42 in C2C12 cells that express Bnip-2 siRNA (+) or an irrelevant sequence (−) in GM or DM were assessed as described in A. (D) Lysates of C2C12 cells cultured in GM to ∼80% confluence and then transferred to DM for the indicated times were immunoprecipitated with Cdo antibodies and then Western blotted with Cdc42 or Cdo antibodies. (E) Levels of GTP-bound and total Cdc42 in C2C12 cells that express Cdo siRNA (+) or an irrelevant sequence (−) in GM or DM were assessed as described in A. The arrowhead indicates a nonspecific band. (F) Levels of GTP-bound and total Cdc42 in myoblasts of the indicated Cdo genotype cultured in GM or DM assessed as described in A.
Because Cdo binds Bnip-2 and Bnip-2 binds Cdc42, we asked whether Cdc42 was present in Cdo immunoprecipitates from differentiating C2C12 cell lysates. Indeed, Cdc42 coprecipitated with Cdo with a maximal interaction 1 d after transfer of cultures to DM (Fig. 6 D). This is similar to Cdo's interaction with Bnip-2 and suggests that formation of a Cdo–Bnip-2–Cdc42 complex promotes Cdc42 activation. To test this possibility further, C2C12 cells that express Cdo siRNA and cultured Cdo−/− myoblasts were analyzed for Cdc42 activity. In C2C12 cells that express Cdo siRNA, the DM-associated elevation in GTP-bound Cdc42 levels seen in control transfectants was almost completely blunted (Fig. 6 E). Primary myoblasts must be cultured in GM plus basic FGF to remain in a proliferative nondifferentiated state, and both Cdo+/+ and Cdo−/− myoblasts had abundant GTP-bound Cdc42 in this medium, likely because of the added growth factor; however, when transferred to DM, the Cdo−/− cells had a lower concentration of active Cdc42 than did the Cdo+/+ cells (Fig. 6 F).
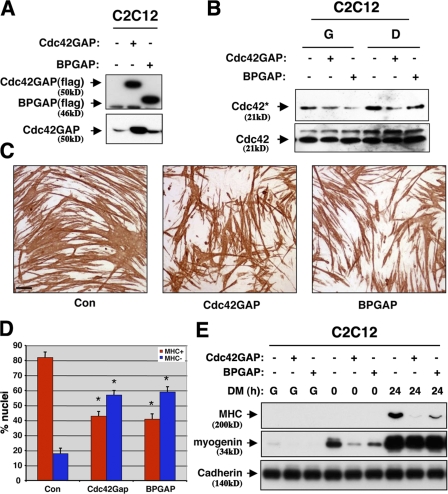
Both Cdo and Bnip-2 positively regulate myogenic differentiation and promote activation of Cdc42, suggesting that Cdc42 is itself promyogenic. However, previous studies in which constitutively active and dominant-negative mutants of Cdc42 were expressed in myoblasts failed to provide evidence for this notion, as both mutants blocked differentiation (Meriane et al., 2000). Expression of siRNA against Cdc42 in C2C12 cells also inhibited differentiation, but these cells acquired a strongly altered morphology (unpublished data). Therefore, to gain information on the role of Cdc42 in myogenesis, we sought to alter Cdc42 activity in a more subtle manner. It was reasoned that the concentration of GTP-bound Cdc42 and, perhaps more importantly, the amount of time Cdc42 proteins spend in the active state could be altered by modulating the amount of Cdc42GAP protein expressed by C2C12 cells. Two GAP proteins for Cdc42, Cdc42GAP and BPGAP (Shang et al., 2003), were each stably overexpressed in C2C12 cells (Fig. 7 A). In both cases, this resulted in lower steady-state levels of GTP-bound Cdc42 in both GM and DM, relative to control vector transfectants (Fig. 7 B). When cultured in DM, C2C12/Cdc42GAP cells and C2C12/BPGAP cells each displayed a similar phenotype: relative to control cells, a smaller percentage of cell nuclei were found in MHC+ myotubes, and the myotubes that formed were shorter and thinner (Fig. 7, C and D). Furthermore, overexpression of Cdc42GAP or BPGAP resulted in delayed induction of the differentiation markers myogenin and MHC (Fig. 7 E).
Figure 7.
Overexpression of Cdc42GAP or BPGAP reduces myogenic differentiation. (A) Lysates of C2C12 cells stably transfected with a control expression vector lacking a cDNA (−) or with expression vectors harboring flag-tagged Cdc42GAP or BPGAP cDNAs as indicated (+) were Western blotted with flag epitope or Cdc42GAP antibodies. (B) Levels of GTP-bound and total Cdc42 in C2C12/Cdc42GAP, C2C12/BPGAP, or vector control (−) cells in GM or DM were assessed as described in Fig. 6 A. (C) Photomicrographs of C2C12/Cdc42GAP, C2C12/BPGAP, and vector control cells that were cultured in DM, fixed, and stained with an antibody to MHC. Bar, 0.1 mm. (D) Quantification of myotube formation. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Asterisks indicate difference from control, P < 0.01. A level of myotube formation by control cells (∼80% nuclei in MHC+ cells) was selected so as to permit visualization of diminished differentiation by Cdc42GAP proteins. (E) Western blot analysis of muscle-specific proteins by C2C12 cell transfectants cultured in GM (G) or in DM for the indicated times.
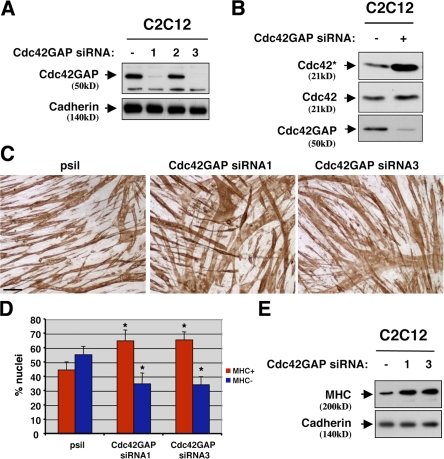
To enhance Cdc42 activity, three independent siRNA sequences against Cdc42GAP were stably expressed in C2C12 cells. Two of these sequences effectively diminished Cdc42GAP protein levels, and this was associated with an increase in the amount of GTP-bound Cdc42 in these cells (Fig. 8, A and B). When cultured in DM, cells with diminished levels of Cdc42GAP produced larger myotubes (Fig. 8 C), had a higher percentage of nuclei present in MHC+ cells (Fig. 8 D), and expressed more MHC protein than did control transfectants (Fig. 8 E). Collectively, the results indicate that Cdc42 activity promotes myogenesis.
Figure 8.
RNAi-mediated depletion of Cdc42GAP enhances myogenic differentiation. (A) Lysates of C2C12 cells stably transfected with pSilencer containing one of three independent Cdc42GAP siRNA sequences (designated 1, 2, and 3) or pSilencer containing an irrelevant sequence (−) were Western blotted with Cdc42GAP or, as a control, pan-cadherin antibodies. (B) Levels of GTP-bound and total Cdc42 in C2C12 cells that express Cdc42GAP siRNA (+) or an irrelevant sequence (−) in DM were assessed as described in Fig. 6 A. (C) Photomicrographs of C2C12 cells that express Cdc42GAP siRNA sequences or an irrelevant (pSilencer) sequence as indicated, cultured in DM, fixed, and stained with an antibody to MHC. Bar, 0.1 mm. (D) Quantification of myotube formation. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Asterisks indicate difference from pSilencer control, P < 0.02. A level of myotube formation by control cells (∼45% nuclei in MHC+ cells) was selected so as to permit visualization of enhanced differentiation by Cdc42GAP siRNA. (E) Western blot analysis of MHC expression by C2C12 cells that express Cdc42GAP siRNA sequences numbers 1 or 3 or an irrelevant sequence (−), cultured in DM for 48 h.
Cdo brings JLP and Bnip-2 together to regulate p38α/β activity
Cdc42 signaling activates p38α/β MAPK in several cell systems (Coso et al., 1995; Minden et al., 1995; Molnár et al., 1997; Bourdoulous et al., 1998). The ability of Cdo to bind Bnip-2 and JLP, which in turn bind Cdc42 and p38α/β, respectively, suggests that Cdo might coordinate Cdc42→p38α/β signaling. The possibility that Bnip-2 and JLP bind the same Cdo complexes was tested initially. COS7 cells were transfected with expression vectors encoding S epitope–tagged JLP (JLP-S), JLP-S and Bnip-2, or JLP-S, Bnip-2, and Cdo. Cell lysates were then precipitated with anti–S agarose and blotted with antibodies to each protein (Fig. 9 A). JLP coprecipitated Bnip-2 in the presence, but not the absence, of coexpressed Cdo. Therefore, JLP and Bnip-2 did not directly interact but were brought together by a mutual interaction with Cdo. To assess whether such complexes form endogenously, lysates of Cdo+/+ and Cdo−/− myoblasts were immunoprecipitated with antibodies to Cdo or to Bnip-2 and then Western blotted with antibodies to Cdo, Bnip-2, and JLP. As expected, Bnip-2 and JLP both coprecipitated with Cdo (Fig. 9 B; Takaesu et al., 2006). Furthermore, JLP coprecipitated with Bnip-2 from Cdo+/+ cell lysates but not Cdo−/− cell lysates, indicating that Bnip-2 and JLP associate in myoblasts in a Cdo-dependent manner (Fig. 9 C).
Figure 9.
Bnip-2 and JLP bind the same Cdo complexes, and Bnip-2 regulates p38α/β activity to promote myogenesis. (A) Lysates of COS7 cells transiently transfected with JLP-S, Bnip-2, Cdo, or control (−) expression vectors as indicated were immunoprecipitated (IP) with anti–S agarose and then Western blotted with JLP, Bnip-2, or Cdo antibodies. (B) Lysates of myoblasts of the indicated Cdo genotype cultured in DM for 48 h were immunoprecipitated with Cdo antibodies and then Western blotted with Cdo, Bnip-2, or JLP antibodies. Total cell lysates were also Western blotted with Bnip-2 or JLP antibodies. (C) Lysates of myoblasts of the indicated Cdo genotype cultured in DM for 48 h were immunoprecipitated with Bnip-2 antibodies and then Western blotted with Cdo, Bnip-2, or JLP antibodies. Total cell lysates were also Western blotted with Cdo or JLP antibodies. The Cdo band below the one indicated by the arrow is a partial degradation product. (D) Lysates of C2C12/Bnip-2 (+) or vector control (−) cells in GM or cultured for 48 h in DM were Western blotted with anti–phospho p38α/β (pp38) or p38α/β (p38) antibodies. (E) Lysates of C2C12 cells stably expressing a Bnip-2 siRNA sequence (+) or an irrelevant sequence (−) in GM or cultured for 48 h in DM were Western blotted with pp38 or p38 antibodies. (F) Lysates of C2C12 cells stably expressing a Cdc42Gap siRNA sequence (+) or an irrelevant sequence (−) in GM or cultured for 48 h in DM were Western blotted with pp38 or p38 antibodies. As the lanes were somewhat unevenly loaded in F, the pp38 and p38 signals were quantified by densitometry, and the pp38/p38 ratio reported under each lane in arbitrary units with the control transfectants in GM set to 1. (G) C2C12 cells were cotransfected with control, Bnip-2 siRNA, or Bnip-2 deletion mutant expression vectors, control or MKK6(EE) expression vectors, and pQ-lacZ (a vector that drives expression of nuclear-localized β-gal to mark transfectants). Differentiated cultures were double-stained for β-gal activity (blue) and for MHC expression (brown). Bar, 0.1 mm. (H) Quantification of C2C12 cell differentiation shown in G. Cultures were scored as MHC− or MHC+, with MHC+ cells further scored as having a single (1) nucleus, two to five nuclei, or greater than five nuclei. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results.
C2C12/Bnip-2 cells, which had elevated Cdc42 activity in GM and DM (Fig. 6), also had elevated levels of the dually phosphorylated (active) form of p38α/β (pp38α/β) in GM and DM (Fig. 9 D). Conversely, C2C12 cells that stably express Bnip-2 siRNA, which poorly activated Cdc42 in response to DM (Fig. 6), were also deficient in DM-induced pp38α/β production (Fig. 9 E). Furthermore, expression of Cdc42GAP siRNA increased pp38α/β levels in C2C12 cells in both GM and DM (Fig. 9 F). Collectively, these results are consistent with a model in which Cdo brings Bnip-2 and JLP together to coordinate Cdc42→p38α/β signaling.
To assess whether p38α/β is functionally downstream of Bnip-2, we asked whether coexpression of the p38α/β activator MKK6(EE) could rescue the differentiation defect associated with expression of Bnip-2 siRNA. The transient C2C12 cell myogenesis assay described earlier (Fig. 5) was used in this case. Approximately ∼83% of double control-vector transfectants were MHC+, and these cells were categorized as those that were mononucleated (∼35% of total cell nuclei), those that had between two and five nuclei (∼45%), and those that had greater than five nuclei (<5%; Fig. 9, G and H). The expression of Bnip-2 siRNA decreased the percentage of MHC+ cells to ∼66%, with multinucleated cells representing only ∼16%, none of which were in the greater-than-five-nuclei category. Therefore, transient expression of Bnip-2 siRNA reduced myogenesis, which is similar to stable knockdown of Bnip-2. Expression of MKK6(EE) alone enhanced myogenesis relative to control transfectants, resulting in ∼94% MHC+ cells, with ∼30% of the total having greater than five nuclei. Cells that coexpressed MKK6(EE) and Bnip-2 siRNA underwent robust myogenesis, though not quite to the level observed with expression of MKK6(EE) alone (∼95% MHC+ cells, but only ∼15% with greater than five nuclei). These results are consistent with the notion that Bnip-2's promyogenic function is exerted mainly, though perhaps not exclusively, via activation of p38α/β.
We also assessed the effects of MKK6(EE) expression on cells that coexpressed the Bnip-2 deletion mutants Δ264–284 and Δ285–292. Similar to the results shown in Fig. 5, expression of either of these two mutants did not dramatically alter myogenesis relative to control transfectants, suggesting a loss of function (Fig. 9, G and H). Coexpression of MKK6(EE) enhanced differentiation of cells expressing these mutants, but the effect was seen more in the production of multinucleated MHC+ cells, with a smaller effect on the percentage of MHC+ versus MHC− cells. The results with the deletion mutants plus or minus MKK6(EE) are somewhat distinct from the effects seen with Bnip-2 siRNA plus or minus MKK6(EE) and suggest that these mutants may have a subtle inhibitory effect made apparent by coexpression of MKK6(EE).
Cdo/Bnip-2 signaling does not account for all p38α/β activity in differentiating myoblasts, and other p38α/β-activating stimuli function in its absence
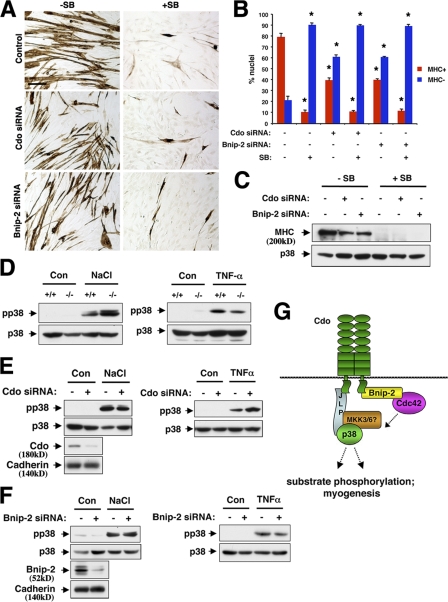
C2C12 cells that express siRNA against Bnip-2 or Cdo, and Cdo−/− myoblasts, each display a partially defective differentiation program accompanied by lower than normal levels of DM-induced pp38α/β (Takaesu et al., 2006; this study). However, myoblasts cultured in the presence of the p38α/β inhibitor SB203580 have a more dramatic blockade to differentiation (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000). It seems likely, therefore, that Cdo/Bnip-2–independent pathways also contribute to p38α/β activation during myogenesis. To examine this point more closely, C2C12 cells that stably express Bnip-2 or Cdo siRNA were treated with SB203580 and assessed for myotube formation and expression of MHC (Fig. 10, A–C). The percentages of control cell nuclei that were present in MHC+ versus MHC− cells was ∼80:20, whereas this MHC+/MHC− ratio was ∼10:90 in SB203580-treated control cells. The MHC+/MHC− ratio of C2C12 cells expressing either Cdo or Bnip-2 siRNA was ∼40:60, which is consistent with incomplete inhibition of differentiation. Treatment of such cells with SB203580 further reduced the ratio to ∼10/90, which is similar to that of vector control cells. Therefore, the cells with diminished levels of Cdo or Bnip-2 that express MHC remain dependent on residual p38α/β activity to do so. Although the extent of siRNA-mediated depletion is not 100%, ∼40% of Cdo−/− myoblasts, which express no Cdo protein, are also MHC+ (Takaesu et al., 2006). These results suggest that other pathways that activate p38α/β are functional in Cdo- and Bnip-2–depleted myoblasts.
Figure 10.
Other mechanisms of p38α/β activation occur in the absence Cdo/Bnip-2 signaling. (A) Photomicrographs of C2C12 cells stably transfected with control vector or vectors expressing Cdo or Bnip-2 siRNA that were cultured in DM plus SB203580 (+SB) or DMSO vehicle (−SB), fixed, and stained with an antibody to MHC. Bar, 0.1 mm. (B) Quantification of the percentage of nuclei in cultures shown in A that were in MHC+ or MHC− cells. Control vector cells are indicated by the negative sign for both Cdo and Bnip-2 siRNA. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Asterisks indicate difference from vector control, P < 0.01. (C) Western blot analysis of cultures shown in A. Extracts were probed with antibodies to MHC or total p38α/β (p38). (D) Myoblasts of the indicated Cdo genotype were treated with 0.9 M NaCl or 10 ng/ml TNFα for 15 min and analyzed for pp38α/β and p38α/β. (E) C2C12 cells stably expressing siRNA against Cdo were treated with NaCl or TNFα and analyzed as in D. (F) C2C12 cells stably expressing siRNA against Bnip-2 were treated with NaCl or TNFα and analyzed as in D. (G) Model of Cdo-mediated p38α/β activation during myogenic differentiation. Cdo binds directly to JLP and, via JLP, to p38α/β. Cdo also binds to Bnip-2 and, via Bnip-2, Cdc42. Formation of a Cdo–Bnip-2–Cdc42 complex promotes or stabilizes activation of Cdc42, which in turn triggers signals culminating in phosphorylation and activation of p38α/β bound to Cdo via JLP. Cdo interacts with itself (Kang et al., 2003) and is shown as a dimer. JLP and Bnip-2 are shown as binding to different Cdo proteins of the dimer for convenience and does not preclude the possibility that JLP and Bnip-2 bind the same Cdo protein simultaneously. See text for additional details.
To assess directly whether additional stimuli can activate p38α/β in cells depleted of Cdo or Bnip-2, Cdo−/− myoblasts and C2C12 cells expressing Cdo or Bnip-2 siRNA were treated with TNFα or with hyperosmotic stress and analyzed for production of pp38α/β. Such cells produced pp38α/β at levels similar to those of control cells in response to these stimuli (Fig. 10, D–F). Collectively, the results suggest that Cdo/Bnip-2 signaling is neither the sole mechanism by which p38α/β becomes activated during myogenesis nor a general requirement for full p38α/β activation but is likely to be specific for differentiation-mediated signaling.
Discussion
Many ubiquitous signaling pathways perform cell type–specific functions. Furthermore, such pathways can regulate disparate functions in the same cell type. Clearly, this requires that the activation of these pathways occurs at the correct time and subcellular location and with the appropriate magnitude and duration. The mechanisms by which such specificity is achieved are not well understood. We previously reported that during myogenic differentiation, the cell surface receptor Cdo binds the p38α/β pathway scaffold protein JLP and, via JLP, p38α/β itself (Takaesu et al., 2006). Cdo−/− myoblasts are deficient in both p38α/β activity and differentiation capability, and their ability to differentiate is rescued by expression of the p38α/β activator MKK6(EE) (Takaesu et al., 2006); however, these results did not explain how association of JLP/p38α/β with Cdo leads to activation of p38α/β.
In this paper, it is demonstrated that Cdo also associates with the Cdc42 binding protein Bnip-2, identifying a novel linkage between a cell surface receptor and downstream modulation of Cdc42 activity. This interaction stimulates Cdc42 and p38α/β activities and regulates myogenic differentiation. Cdo, therefore, appears to promote activation of p38α/β by assembly of multiprotein signaling modules for Cdc42 and p38α/β via direct binding to scaffold proteins for each (i.e., Bnip-2 and JLP, respectively; Fig. 10 G). JLP and Bnip-2 associate in a Cdo-dependent manner, implying that Cdc42 bound to Cdo via Bnip-2 activates p38α/β bound to Cdo via JLP and that this represents a pool of p38α/β specifically activated during differentiation. We speculate that binding of Bnip-2–Cdc42 to Cdo allows Cdc42 to interact with a specific guanine nucleotide exchange factor, promoting nucleotide exchange on Cdc42 and subsequent binding to effector proteins that initiate a kinase cascade resulting in activation of p38α/β. Alternatively, Cdc42 may be activated before association with Cdo, and subsequent interaction between Cdo, Bnip-2, and Cdc42 stabilizes Cdc42 in the active state and brings it into proximity with components of the p38α/β pathway.
All the described components of this complex are ubiquitously expressed except Cdo, which, though not muscle specific, is highly enriched in muscle precursor cells and differentiating muscle (Kang et al., 1998; Mulieri et al., 2000), suggesting that Cdo itself may provide some level of cell type specificity to this signaling pathway. Cdo is also expressed at high levels in neuronal precursors, and similar results to those reported here have been obtained in experiments on neuronal differentiation of a neural precursor line (unpublished observations). It is also clear, however, that not all p38α/β pathway activity during myoblast differentiation is Cdo- or Bnip-2–dependent, as residual pp38α/β is detected in cells depleted for Cdo or Bnip-2, and treatment of such cells with the p38α/β inhibitor SB203580 further inhibits their differentiation. Potential additional mechanisms for activation of p38α/β in differentiating myoblasts include low-level autocrine TNFα signaling and signaling by semaphorin 4C (Ko et al., 2005; Chen et al., 2007; Riuzzi et al., 2007; Wu et al., 2007).
In addition to binding Cdc42, Bnip-2 binds its negative regulator, Cdc42GAP (Low et al., 1999). However, loss- and gain-of-function experiments in myoblasts indicate that Bnip-2 stimulates Cdc42 activity. These data suggest that Bnip-2 may function as a scaffold for dynamic signaling through Cdc42, modulating the balance or kinetics of its activity cycle. This is the first paper to find an endogenous function for Bnip-2, and we are unaware of another protein with this type of activity for Cdc42. Active cycling of Cdc42 may be required for efficient myogenesis, as the results presented in this paper demonstrate that Cdc42 activity is important for this process, but expression of constitutively active or dominant-negative mutants of Cdc42 each block differentiation (Meriane et al., 2000). The ability of Cdc42 to trigger p38α/β activation fits well with the known importance of p38α/β in myogenic differentiation (Lluis et al., 2006). However, Cdc42 regulates additional processes that are also likely to be relevant to differentiation, such as the formation of filopodia, which may be required for cell–cell interactions in preparation for fusion, and regulation of cell polarity (Jaffe and Hall, 2005). That forced expression of the p38α/β activator MKK6(EE) has a somewhat greater effect on differentiation of control myoblasts than on myoblasts depleted of Bnip-2 is consistent with the notion that promyogenic pathways other than p38α/β lie downstream of Cdc42, but additional work will be required to establish that this is the case.
Collectively, these results reveal a distinctive mechanism of signaling: the Cdo intracellular region binds to scaffold proteins that in turn bind multiple components of specific pathways. This is different from other Ig/FNIII repeat receptors related to Cdo, such as the Robo proteins, which bind to the nonreceptor tyrosine kinase, c-Abl, the Rho family GAPs srGAP and Vilse, the SH2/SH3 adaptor Nck, and the actin regulator Ena/Vasp (Dickson and Gilestro, 2006). Furthermore, Cdo functions in multiple contexts at the extracellular face of the cell surface. In myoblasts, it forms cis complexes with N-cadherin and neogenin, the latter a receptor for the netrin and RGM families of ligands (Kang et al., 2003, 2004; Cole et al., 2007). Additionally, Cdo binds Sonic hedgehog, possibly as a coreceptor with Patched1 (Tenzen et al., 2006; Yao et al., 2006; Martinelli and Fan, 2007). The mechanism by which binding of JLP and Bnip-2 to Cdo is induced during myogenesis is not clear, but a connection with N-cadherin is likely. Expression in C2C12 cells of a Cdo deletion mutant that is specifically deficient in its ability to bind N-cadherin blocks differentiation (Kang et al., 2003). N-cadherin–based adhesion enhances p38α/β activity in C2 myoblasts (Lovett et al., 2006), and preliminary results suggest that Cdo, JLP, and Bnip-2 can be recruited to sites of N-cadherin ligation (unpublished data). Cdo may function to link cadherin-based adhesion to the p38α/β pathway, which promotes the muscle-specific transcriptional program. That Cdc42, a known regulator of actin dynamics, is directly involved in this process provides a mechanism by which such changes in gene expression can be coordinated with the alterations in cell morphology that are also required for cell differentiation.
Materials and methods
Yeast two-hybrid screen
Bnip-2 was identified in a previously reported screen in which the transmembrane plus intracellular region of mouse Cdo was used as bait (Takaesu et al., 2006). Two independent clones were isolated a total of eight times. Both clones started in the 5′ UTR, were in frame, and encoded the entire Bnip-2 ORF.
Cell culture
C2C12, 293T, COS7, and myoblasts derived from Cdo+/+ and Cdo−/− mice were cultured as previously described (Kang et al., 1998, 2004; Cole et al., 2004). To induce differentiation of C2C12 cells, cultures were transferred from DME containing 15% FBS (GM) to DME containing 2% horse serum (DM). Myotube formation in stable and transient assays was performed and quantified as previously described (Kang et al., 2004). Statistical analysis of the number of nuclei in MHC+ versus MHC− cells was done with Student's t test.
For stable overexpression studies in myoblasts, pXJ40 vectors encoding flag-tagged forms of Bnip-2, Cdc42GAP, or BPGAP (Low et al., 1999, 2000a,b) were cotransfected with pBabePuro (Morgenstern and Land, 1990) into C2C12 cells with FuGene6 (Roche), and cultures were selected in puromycin-containing medium. Drug-resistant cells were pooled and analyzed. Multiple such pools were studied in each case.
For siRNA studies, the following sequences were inserted into pSilencer 2.1-U6 hygro (Ambion): Bnip-2 #1: 5′-GATCCCGATCAGATACGTCTTTAACTTCAAGAGAGTTAAAGACGTATCTGATCGTTTTTTGGAAA-3′; Bnip-2 #3: 5′-GATCCCGGAAGAATGGCAGGATGAATTCAAGAGATTCATCCTGCCAT TCTTCCGTTTTTTGGAAA-3′; Bnip-2 #4: 5′-GATCCCGTGGTGCGACAACTCGAAGATTCAAGAGATCTTCGAGTTGTCGCACCACGTTTTTTGGAAA-3′; Cdc42GAP #1: 5′-GATCCCGGCCAAGCTCTGAACCAGTTTTCAAGAGAAACTGGTTCAGAGCTTGGCTTTTTTGGAAA-3′; and Cdc42GAP #3: 5′-GATCCCGCCATCACCCTCAAGGCTATTTCAAGAGAATATAGCCTTGAGGGTGATGGTTTTTTGGAAA-3′. Cdo siRNA sequence #1 (Zhang et al., 2006) was used in similar fashion. The pSilencer 2.1-U6 hygro vector harboring an irrelevant sequence (Ambion) was used as a control. These vectors were transfected into C2C12 cells with FuGene6 and cultures were selected in hygromycin-containing medium. Drug-resistant cells were pooled and analyzed. Multiple such pools were studied in each case.
Where indicated, Cdo+/+ and Cdo−/− myoblasts and C2C12 cell derivatives were treated with 10 ng/ml TNFα (Sigma-Aldrich) or 0.9 M NaCl for 15 min. C2C12 cell derivatives were treated with 2.5 μM SB203580 (EMD) in DM, replenished every 12 h, and harvested after 48 h for analysis.
For Cdo–Bnip-2 interaction studies, expression vectors encoding Cdo, myc-tagged Boc, Bnip-2, and Bnip-2 deletion mutants (Low et al., 2000b; Kang et al., 2002; Zhou et al., 2005) were transiently transfected into COS7 or 293T cells with FuGene6 or calcium phosphate, respectively. 48 h later, cells were harvested for Western blot and immunoprecipitation analyses as described in the next section.
Western blot, immunoprecipitation, and Cdc42 activity analyses
Western blot analyses were performed as previously described (Kang et al., 2004). For immunoprecipitations, cells were lysed in extraction buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, and 0.5% Triton X-100 supplemented with one tablet/40 ml of Complete protease inhibitor cocktail [Roche]). 1 mg of whole cell extract from each sample was precleared with protein G–Sepharose (GE Healthcare) conjugated with 1 μg of normal rabbit IgG (Santa Cruz Biotechnology, Inc.) for 1 h at 4°C, followed by immunoprecipitation with 1 μg of Cdo, flag epitope, or Bnip-2 antibodies for 2 h at 4°C. Immunocomplexes were washed three times with, and suspended in, extraction buffer, and samples were analyzed by Western blotting. For S-agarose pulldown experiments, whole cell extracts were incubated with 20 μl of 50% slurry S-protein agarose beads (EMD) for 3.5 h at 4°C. Beads were washed three times with, and suspended in, extraction buffer, and samples were analyzed by Western blotting. Levels of GTP-bound Cdc42 were analyzed with the Cdc42 Activation Assay kit with PAK-1 PBD-agarose (Millipore), according to the manufacturer's instructions.
Antibodies used were the following: anti-Cdo (Invitrogen), anti-p38α/β (Sigma-Aldrich), anti-pp38α/β (Cell Signaling Technology), anti–S-probe (Santa Cruz Biotechnology, Inc.), anti-MHC (MF-20; Developmental Studies Hybridoma Bank), anti-TnT (Sigma-Aldrich), anti-myogenin (Santa Cruz Biotechnology, Inc.), anti-Cdc42 (Millipore), anti-Cdc42GAP (Abnova), anti–Bnip-2 (Low et al., 1999), anti-flag epitope (Sigma-Aldrich), anti–pan-cadherin (Sigma-Aldrich), anti-Boc, and anti-myc (9E10; Mount Sinai Hybridoma Core Facility).
Microscopy
Cultures were fixed and processed for MHC expression and/or β-gal activity as described in the previous sections and examined on a phase contrast microscope (Eclipse TS100; Nikon) with Plan Fluor 10×/0.3 NA and 20×/0.45 NA objectives (Nikon) at room temperature. Images were captured with a camera (Spot RT Color model 2.2.1; Diagnostic Instruments, Inc.) using Spot software (version 3.5.9; Diagnostic Instruments, Inc.) and Photoshop 7.0 (Adobe).
Acknowledgments
We thank Reshma Taneja, Jeanne Hirsch, and members of the Krauss laboratory for critical reading of the manuscript and David Glass for helpful discussions.
This work was supported by grants from the National Institutes of Health (AR46207) and the T.J. Martell Foundation to R.S. Krauss, the Samsung Biomedical Research Institute (B-A7-002) to J.S. Kang, and the Biomedical Reasearch Council Singapore (R154000271305) to B.C. Low.
J.-S. Kang and G.-U. Bae contributed equally to this paper.
G. Takaesu's present address is Division of Molecular and Cellular Immunology, Medical Institute of Bioregulation, Kyushu University, Fukuoka 812-8582, Japan.
Abbreviations used in this paper: β-gal, β-galactosidase; DM, differentiation medium; GAP, GTPase-activating protein; GM, growth medium; MHC, myosin heavy chain.
References
- Bourdoulous, S., G. Orend, D.A. MacKenna, R. Pasqualini, and E. Ruoslahti. 1998. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J. Cell Biol. 143:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briata, P., S.V. Forcales, M. Ponassi, G. Corte, C.Y. Chen, M. Karin, P.L. Puri, and R. Gherzi. 2005. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell. 20:891–903. [DOI] [PubMed] [Google Scholar]
- Chen, S.E., B. Jin, and Y.P. Li. 2007. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 292:C1660–C1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, F., W. Zhang, A. Geyra, J.-S. Kang, and R.S. Krauss. 2004. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell. 7:843–854. [DOI] [PubMed] [Google Scholar]
- Cole, S.J., D. Bradford, and H.M. Cooper. 2007. Neogenin: a multi-functional receptor regulating diverse developmental processes. Int. J. Biochem. Cell Biol. 39:1569–1575. [DOI] [PubMed] [Google Scholar]
- Coso, O.A., M. Chiariello, J.-C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J.S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 81:1137–1146. [DOI] [PubMed] [Google Scholar]
- Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274:4341–4346. [DOI] [PubMed] [Google Scholar]
- Dickson, B.J., and G.F. Gilestro. 2006. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 22:651–675. [DOI] [PubMed] [Google Scholar]
- Gallo, R., M. Serafini, L. Castellani, G. Falcone, and S. Alemà. 1999. Distinct effects of Rac1 on differentiation of primary avian myoblasts. Mol. Biol. Cell. 10:3137–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, A.B., and A. Hall. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269. [DOI] [PubMed] [Google Scholar]
- Jones, N.C., K.J. Tyner, L. Nibarger, H.M. Stanley, D.D. Cornelison, Y.V. Fedorov, and B.B. Olwin. 2005. The p38α/β MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 169:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.-S., P.J. Mulieri, C. Miller, D.A. Sassoon, and R.S. Krauss. 1998. CDO, a Robo-related cell surface protein that mediates myogenic differentiation. J. Cell Biol. 143:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.-S., P.J. Mulieri, Y. Hu, L. Taliana, and R.S. Krauss. 2002. BOC, an Ig superfamily member, associates with CDO to positively regulate myogenic differentiation. EMBO J. 21:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.-S., J.L. Feinleib, S. Knox, M.A. Ketteringham, and R.S. Krauss. 2003. Pro-myogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc. Natl. Acad. Sci. USA. 100:3989–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.-S., M.-J. Yi, W. Zhang, J.L. Feinleib, F. Cole, and R.S. Krauss. 2004. Netrins and neogenin promote myotube formation. J. Cell Biol. 167:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, J.A., T. Gondo, S. Inagaki, and M. Inui. 2005. Requirement of the transmembrane semaphorin Sema4C for myogenic differentiation. FEBS Lett. 579:2236–2242. [DOI] [PubMed] [Google Scholar]
- Lluis, F., E. Ballestar, M. Suelves, M. Esteller, and P. Munoz-Canoves. 2005. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 24:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis, F., E. Perdiguero, A.R. Nebreda, and P. Munoz-Canoves. 2006. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 16:36–44. [DOI] [PubMed] [Google Scholar]
- Lovett, F.A., I. Gonzalez, D.A. Salih, L.J. Cobb, G. Tripathi, R.A. Cosgrove, A. Murrell, P.J. Kilshaw, and J.M. Pell. 2006. Convergence of Igf2 expression and adhesion signalling via RhoA and p38 MAPK enhances myogenic differentiation. J. Cell Sci. 119:4828–4840. [DOI] [PubMed] [Google Scholar]
- Low, B.C., Y.P. Lim, J. Lim, E.S. Wong, and G.R. Guy. 1999. Tyrosine phosphorylation of the Bcl-2-associated protein BNIP-2 by fibroblast growth factor receptor-1 prevents its binding to Cdc42GAP and Cdc42. J. Biol. Chem. 274:33123–33130. [DOI] [PubMed] [Google Scholar]
- Low, B.C., K.T. Seow, and G.R. Guy. 2000. a. Evidence for a novel Cdc42GAP domain at the carboxyl terminus of BNIP-2. J. Biol. Chem. 275:14415–14422. [DOI] [PubMed] [Google Scholar]
- Low, B.C., K.T. Seow, and G.R. Guy. 2000. b. The BNIP-2 and Cdc42GAP homology domain of BNIP-2 mediates its homophilic association and heterophilic interaction with Cdc42GAP. J. Biol. Chem. 275:37742–37751. [DOI] [PubMed] [Google Scholar]
- Martinelli, D.C., and C.M. Fan. 2007. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 21:1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriane, M., S. Charrasse, F. Comunale, A. Mery, P. Fort, P. Roux, and C. Gauthier-Rouviere. 2000. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell. 11:2513–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden, A., A. Lin, F.-X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcription activity by the small GTPases Rac and Cdc42Hs. Cell. 81:1147–1157. [DOI] [PubMed] [Google Scholar]
- Molnár, A., A.M. Theodoras, L.I. Zon, and J.M. Kyriakis. 1997. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J. Biol. Chem. 272:13229–13235. [DOI] [PubMed] [Google Scholar]
- Morgenstern, J.P., and H. Land. 1990. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulieri, P.J., A. Okada, D.A. Sassoon, S.K. McConnell, and R.S. Krauss. 2000. Developmental expression pattern of the cdo gene. Dev. Dyn. 219:40–49. [DOI] [PubMed] [Google Scholar]
- Perdiguero, E., V. Ruiz-Bonilla, L. Gresh, L. Hui, E. Ballestar, P. Sousa-Victor, B. Baeza-Raja, M. Jardí, A. Bosch-Comas, M. Esteller, et al. 2007. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38α in abrogating myoblast proliferation. EMBO J. 26:1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall, M.E., M.K. Gustafsson, and C.P. Emerson Jr. 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18:747–783. [DOI] [PubMed] [Google Scholar]
- Puri, P.L., Z. Wu, P. Zhang, L.D. Wood, K.S. Bhakta, J. Han, J.R. Feramisco, M. Karin, and J.Y.J. Wang. 2000. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma. Genes Dev. 14:574–584. [PMC free article] [PubMed] [Google Scholar]
- Riuzzi, F., G. Sorci, and R. Donato. 2007. RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth. Am. J. Pathol. 171:947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, X., Y.T. Zhou, and B.C. Low. 2003. Concerted regulation of cell dynamics by BNIP-2 and Cdc42GAP homology/Sec14p-like, proline-rich, and GTPase-activating protein domains of a novel Rho GTPase-activating protein, BPGAP1. J. Biol. Chem. 278:45903–45914. [DOI] [PubMed] [Google Scholar]
- Shingai, T., W. Ikeda, S. Kakunaga, K. Morimoto, K. Takekuni, S. Itoh, K. Satoh, M. Takeuchi, T. Imai, M. Monden, and Y. Takai. 2003. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J. Biol. Chem. 278:35421–35427. [DOI] [PubMed] [Google Scholar]
- Simone, C., S.V. Forcales, D.A. Hill, A.N. Imbalzano, L. Latella, and P.L. Puri. 2004. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36:738–743. [DOI] [PubMed] [Google Scholar]
- Takaesu, G., J.S. Kang, G.U. Bae, M.J. Yi, C.M. Lee, E.P. Reddy, and R.S. Krauss. 2006. Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 175:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, H., I. Komuro, T. Oka, I. Shiojima, Y. Hiroi, T. Mizuno, and Y. Yazaki. 1998. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 18:1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott, S.J. 2005. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 132:2685–2695. [DOI] [PubMed] [Google Scholar]
- Tenzen, T., B.L. Allen, F. Cole, J.-S. Kang, R.S. Krauss, and A.P. McMahon. 2006. The cell surface membrane proteins Cdo and Boc are components and targets of the hedgehog signaling pathway and feedback network in mice. Dev. Cell. 10:647–656. [DOI] [PubMed] [Google Scholar]
- Travaglione, S., G. Messina, A. Fabbri, L. Falzano, A.M. Giammarioli, M. Grossi, S. Rufini, and C. Fiorentini. 2005. Cytotoxic necrotizing factor 1 hinders skeletal muscle differentiation in vitro by perturbing the activation/deactivation balance of Rho GTPases. Cell Death Differ. 12:78–86. [DOI] [PubMed] [Google Scholar]
- Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295–2322. [DOI] [PubMed] [Google Scholar]
- Wu, H., X. Wang, S. Liu, Y. Wu, T. Zhao, X. Chen, L. Zhu, Y. Wu, X. Ding, X. Peng, et al. 2007. Sema4C participates in myogenic differentiation in vivo and in vitro through the p38 MAPK pathway. Eur. J. Cell Biol. 86:331–344. [DOI] [PubMed] [Google Scholar]
- Wu, Z., P.J. Woodring, K.S. Bhakta, K. Tamura, F. Wen, J.R. Feramisco, M. Karin, J.Y. Wang, and P.L. Puri. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20:3951–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, S., L. Lum, and P. Beachy. 2006. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 125:343–357. [DOI] [PubMed] [Google Scholar]
- Zarubin, T., and J. Han. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. [DOI] [PubMed] [Google Scholar]
- Zetser, A., E. Gredinger, and E. Bengal. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274:5193–5200. [DOI] [PubMed] [Google Scholar]
- Zhang, W., J.-S. Kang, F. Cole, M.J. Yi, and R.S. Krauss. 2006. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell. 10:657–665. [DOI] [PubMed] [Google Scholar]
- Zhou, Y.T., G.R. Guy, and B.C. Low. 2005. BNIP-2 induces cell elongation and membrane protrusions by interacting with Cdc42 via a unique Cdc42-binding motif within its BNIP-2 and Cdc42GAP homology domain. Exp. Cell Res. 303:263–274. [DOI] [PubMed] [Google Scholar]