Abstract
Transforming growth factor β (TGFβ) promotes epithelial cell differentiation but induces Schwann cell proliferation. We show that the protooncogene Ski (Sloan-Kettering viral oncogene homologue) is an important regulator of these effects. TGFβ down-regulates Ski in epithelial cells but not in Schwann cells. In Schwann cells but not in epithelial cells, retinoblastoma protein (Rb) is up-regulated by TGFβ. Additionally, both Ski and Rb move to the cytoplasm, where they partially colocalize. In vivo, Ski and phospho-Rb (pRb) appear to interact in the Schwann cell cytoplasm of developing sciatic nerves. Ski overexpression induces Rb hyperphosphorylation, proliferation, and colocalization of both proteins in Schwann cell and epithelial cell cytoplasms independently of TGFβ treatment. Conversely, Ski knockdown in Schwann cells blocks TGFβ-induced proliferation and pRb cytoplasmic relocalization. Our findings reveal a critical function of fine-tuned Ski levels in the control of TGFβ effects on the cell cycle and suggest that at least a part of Ski regulatory effects on TGFβ-induced proliferation of Schwann cells is caused by its concerted action with Rb.
Introduction
Unlike the central nervous system, peripheral nerves can regenerate efficiently. This ability is largely attributed to Schwann cells, glia cells of the peripheral nervous system that are able to dedifferentiate, proliferate and redifferentiate after injury, foster axonal regrowth, and rebuild myelin sheaths. Schwann cells also constitute a key lineage in nerve development, supporting the survival of neurons and axons as well as providing myelination for efficient saltatory nerve conduction. Thus, understanding the regulatory mechanisms that guide Schwann cell proliferation, apoptosis, differentiation, dedifferentiation, and redifferentiation after injury is of paramount importance for nerve biology in health and disease.
TGFβ is a key factor involved, triggering Schwann cell proliferation or apoptosis, depending on the cell maturation stage (Eccleston et al., 1989; Ridley et al. 1989; Atanasoski et al., 2004; Parkinson et al., 2004; D'Antonio et al., 2006). Interestingly, the same growth factor can induce growth arrest and differentiation of epithelial cells (Schiller et al., 2004). The mechanisms underlying these cell type–specific effects of TGFβ on the cell cycle are largely unknown.
TGFβ is a ubiquitously expressed cytokine that affects crucial biological processes such as proliferation, immunity, and wound healing. Indeed, TGFβ is an antiproliferative agent in various tissues, including epithelial cells, and mutations in its signaling pathway are frequently found in epithelial cancers. TGFβ is also involved in fibrotic diseases including lung fibrosis, liver cirrhosis, hypertrophic scars, and keloids, and the inhibition of its pathway may constitute a treatment for fibrosis.
We have found that the protooncogene Ski (Sloan-Kettering viral oncogene homologue), a crucial negative regulator of TGFβ signaling (Luo, 2004), plays a key role in the control of Schwann cell proliferation and myelination (Atanasoski et al., 2004). In epithelial cells, activation of TGFβ receptors leads to phosphorylation of the signaling proteins Smad2/3. In turn, the latter form a complex with Smad4, translocate to the nucleus, and induce the expression of a specific set of downstream genes. Ski regulates and inactivates this mechanism by binding to Smad2/3. Additionally, Ski action is modulated by its interaction with multiple other partners, including SnoN, c-Jun, retinoic acid receptor, Gli3, histone deacetylase 1, N-CoR, mSin3a, MeCP2, HIPK2, Skip, C184M, NF1, GATA1, and retinoblastoma protein (Rb; Luo, 2004). Rb is of particular interest in this context as a nuclear tumor suppressor regulating the G1/S-phase transition. Its hypophosphorylated form arrests cells in G1 phase by binding to the transcription factor E2F to repress its activity. When hyperphosphorylated, Rb releases E2F. The latter is thus activated and promotes entry into S phase. In vitro studies indicate that c-Ski is required for the transcriptional repression mediated by Rb (Tokitou et al., 1999).
In epithelial cells, TGFβ promotes cycle arrest through down-regulation of c-myc (Pietenpol et al., 1990; Alexandrow et al., 1995), inhibition of Cdk2 (Polyak et al., 1994; Cipriano and Chen, 1998) and Cdk4 (Hannon and Beach, 1994) activities, and inhibition of E2F-dependent transcription (Schwarz et al., 1995; Li et al., 1997; Iavarone and Massague, 1999). The cyclin-dependent kinases Cdk2 and Cdk4/Cdk6 regulate E2F-dependent transcription through phosphorylation of Rb (Horton et al., 1995; Connell-Crowley et al., 1997; Lundberg and Weinberg, 1998). Therefore, by inhibiting Cdk2 and Cdk4 activities in epithelial cells, TGFβ mediates cell cycle arrest by preventing hyperphosphorylation and thus inactivation of Rb.
In Schwann cells, TGFβ does not induce growth arrest and differentiation but, on the contrary, stimulates proliferation. This difference compared with epithelial cells is intriguing and prompted us to search for its molecular basis. We show that in Schwann cells, in contrast to epithelial cells, TGFβ does not alter Ski expression and up-regulates Rb, particularly its hyperphosphorylated form. Furthermore, TGFβ triggers Ski and Rb relocalization as a complex to the cytoplasm most likely to promote TGFβ-induced Schwann cell proliferation. This regulatory mechanism, occurring in Schwann cells but not in epithelial cells, is crucially dependent on the levels of Ski expression.
Results
TGFβ induces Schwann cell proliferation but decreases proliferation in epithelial cells and promotes their differentiation
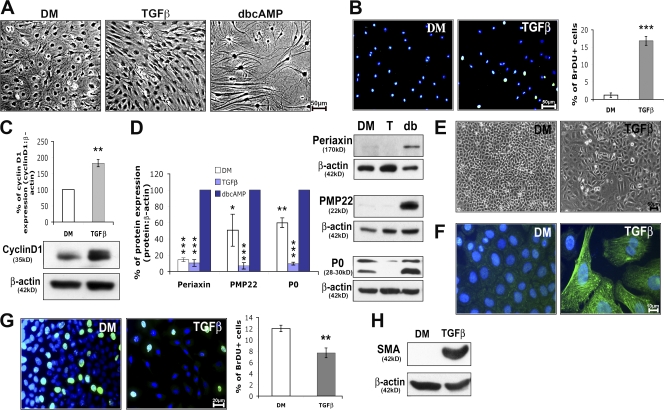
Previous studies indicated that TGFβ is able to induce Schwann cell proliferation (Atanasoski et al., 2004) or apoptosis (Parkinson et al., 2004) in vitro, depending on the culture conditions. In our chosen experimental setting (Fig. 1 A), the addition of TGFβ to growth-arrested Schwann cells in defined medium (DM) strongly promoted proliferation, as shown by increased S-phase entry (Fig. 1 B) and up-regulation of the proliferation marker cyclin D1 (Fig. 1 C). As previously reported (Harrisingh et al., 2004), treatment with dibutyryl cAMP (dbcAMP) induced Schwann cell differentiation, as indicated by up-regulation of the myelin proteins peripheral myelin protein 22 (PMP22), myelin protein zero (P0), and periaxin after 48 h (Fig. 1 D).
Figure 1.
TGFβ induces Schwann cell proliferation but promotes epithelial cell differentiation. (A) Morphology of growth-arrested (cultured in defined medium [DM] alone), proliferating (TGFβ), and differentiated (dbcAMP) rat Schwann cells. (B and G) BrdU incorporation in rat Schwann cells (B) and in WB-F344 cells (G) cultured in DM or treated with TGFβ (BrdU, green; DAPI, blue; double stain, turquoise), and graph representing the percentage of BrdU-positive cells. (C) Western blot analysis of cyclin D1 in lysates of rat Schwann cells cultured in DM (set to 100%) or treated with TGFβ. (B, C, and G) n = 3. (D) Western blot analysis of periaxin, PMP22 (*, P = 0.032; one-tailed t test), and P0 in rat Schwann cells cultured in DM treated with TGFβ (T) or dbcAMP (db; set to 100%). n ≥ 3. (E) Morphology of WB-F344 cells in DM alone or treated with TGFβ. (F) Immunostaining of SMA (green) and DAPI (blue) labeling of WB-F344 cells cultured in DM or treated with TGFβ. (H) Western blot analysis of SMA in WB-F344 cells cultured in DM or treated with TGFβ. For Western blot analyses, β-actin was used as loading control, and graphs represent the densitometry of the protein of interest normalized to the loading control. Statistical analyses were performed using two-tailed t tests on at least three independent experiments, unless mentioned otherwise. Error bars represent SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Rat epithelial cells of the line WB-F344 continued to proliferate when cultured in DM and were not growth arrested like Schwann cells, indicating that growth factors present in DM were sufficient to stimulate their proliferation. As reported previously with other epithelial cell lines, the addition of TGFβ reduced cellular proliferation and promoted cell differentiation (Fig. 1, E–H; Schiller et al., 2004). WB-F344 cells cultured in DM had a polygonal morphology, and their size increased dramatically, whereas they acquired an irregular morphology when treated with TGFβ (Fig. 1 E). Size increase and expression of α smooth muscle actin (SMA) are commonly used as markers of epithelial cell differentiation (Chen et al., 2006; Mikaelian et al., 2006). In DM alone, WB-F344 cells did not express SMA, but the majority of cells strongly expressed this differentiation marker after TGFβ treatment (Fig. 1, F and H). Each experiment was performed at least three times independently, and statistical analyses were performed using two-tailed t tests. We concluded that cultured Schwann cells and WB-F344 cells are suitable for our further studies aimed at identifying the different pathways that lead to cell-specific effects of TGFβ on the cell cycle.
TGFβ down-regulates Ski in epithelial cells but not in Schwann cells
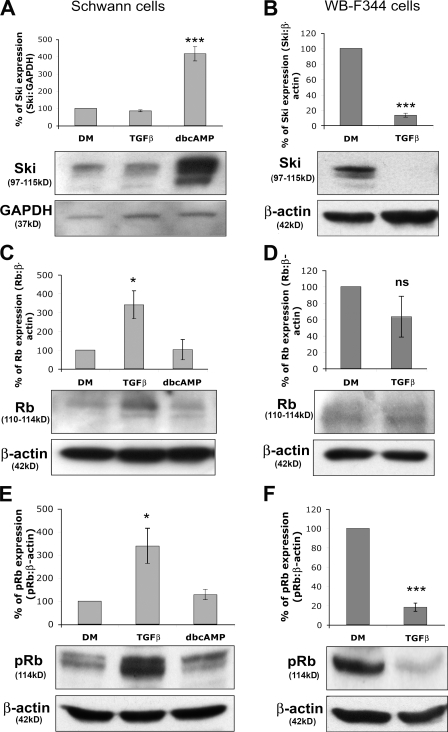
Next, we examined Ski levels in our cell culture systems. In Schwann cells, treatment with TGFβ had no effect on Ski protein endogenous expression, whereas differentiation induced by dbcAMP led to a strong increase (Fig. 2 A). In contrast, and in accordance with previous experiments performed on other epithelial cells (Sun et al., 1999), Ski was drastically down-regulated in TGFβ-treated WB-F344 cells (Fig. 2 B). Each experiment was performed four times independently, and statistical analyses were performed using two-tailed t tests.
Figure 2.
TGFβ down-regulates Ski in epithelial cells but not in Schwann cells and mediates Rb up-regulation in Schwann cells but not in epithelial cells. Western blot analysis of Ski (A), Rb (C), and ser780pRb (pRb; E) in Schwann cells cultured in DM alone or treated with TGFβ or dbcAMP. Western blot analysis of Ski (B), Rb (D), and ser780pRb (pRb; F) in WB-F344 cells cultured in DM or treated with TGFβ. GAPDH or β-actin were used as loading controls. Graphs represent the densitometry analysis of the protein of interest normalized to the loading control. Statistical analyses were performed using two-tailed t tests on at least three independent experiments. Error bars represent SEM. *, P < 0.05; ***, P < 0.001. A and B, n = 4; C, n ≥ 3; D–F, n = 3.
TGFβ up-regulates total Rb and ser780pRb in Schwann cells but down-regulates ser780pRb in epithelial cells
Because of the key role of Rb in the regulation of cell proliferation and its known interaction with Ski, we analyzed Rb in our experimental setting. TGFβ treatment significantly up-regulated total Rb as well as hyperphosphorylated ser780pRb (serine780-phosphorylated Rb) protein levels in proliferating Schwann cells, whereas dbcAMP-induced differentiation had no detectable effect (Fig. 2, C and E). In WB-F344 cells, TGFβ-induced differentiation did not significantly alter total Rb levels (Fig. 2 D) but strongly decreased the level of ser780pRb (Fig. 2 F) in functional agreement with decreased proliferation. Each experiment was performed at least three times independently, and statistical analyses were performed using two-tailed t tests.
TGFβ induces localization of Ski and Rb in the cytoplasm of Schwann cells but not of epithelial cells
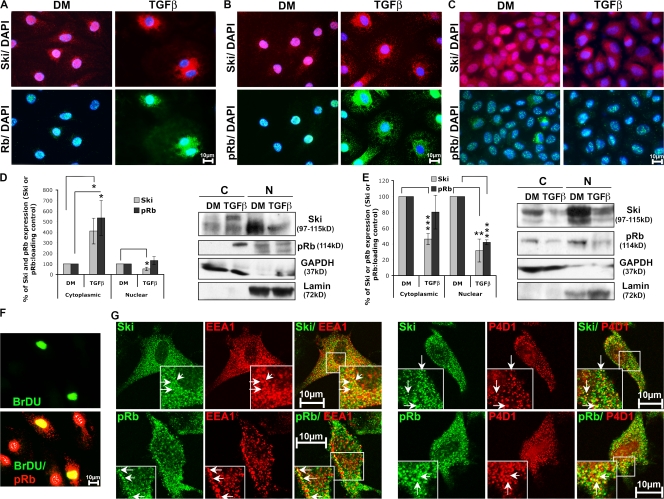
The up-regulation of total Rb and ser780pRb in TGFβ-treated Schwann cells, which is compatible with the observed increased proliferation, was intriguing because it may indicate a potential active functional role of, in particular, the hyperphosphorylated form of Rb in this cell type. To examine this issue further, we analyzed the intracellular localization of Rb in conjunction with Ski by immunofluorescence and cell fractionation methods. In DM-cultured Schwann cells, total Rb (Fig. 3 A), and ser780pRb (Figs. 3, B and D) were almost exclusively found in the nucleus. After 48-h treatment with TGFβ, however, a fraction of the proteins was present in the cytoplasm. This was surprising because Rb is a nuclear regulator of the G1/S-phase transition, and the cytoplasm was an unexpected subcellular localization for this protein. Parallel analysis of Ski localization revealed that Ski was concentrated in the nucleus (with minor amounts occasionally present in the perinuclear region) of DM-cultured Schwann cells, but after TGFβ treatment for 48 h, Ski was mainly found in the cytoplasm (Fig. 3, A, B, and D). A time course analysis after TGFβ treatment showed that both Ski and Rb started to be relocalized into the cytoplasm after 6 h, reaching a maximum after 48 h (unpublished data). In differentiated Schwann cells (after treatment with dbcAMP), Ski and Rb were concentrated in the nucleus, and no cytoplasmic localization was observed (unpublished data). WB-F344 cells showed Ski and ser780pRb expression in both the nucleus and the cytoplasm, and no obvious relocalization was observed after TGFβ treatment (Fig. 3, C and E). We were unable to investigate the subcellular localization of total Rb in WB-F344 cells because of a dramatic change in the quality of the commercially available anti-Rb antibodies and the lack of suitable alternative reagents. Each experiment was performed at least three times independently, and statistical analyses were performed using two-tailed t tests.
Figure 3.
In Schwann cells but not in epithelial cells, TGFβ treatment triggers Ski and Rb localization into the cytoplasm. (A–C) Coimmunostaining of Ski (red) and Rb (green; A) or Ski (red) and ser780pRb (pRb; green; B) in rat Schwann cells or WB-F344 cells (C) cultured in DM or treated with TGFβ for 48 h. Nuclei are labeled with DAPI (blue), and each picture represents the overlay of Ski and DAPI (appears pink when Ski is nuclear), Rb, or ser780pRb and DAPI (appears turquoise when Rb or ser780pRb is nuclear). (D and E) Western blot of Ski and ser780pRb (pRb) in cytoplasmic (C) and nuclear (N) fractions of rat Schwann cells (D) and WB-F344 cells (E) cultured in DM (set to 100%) or treated with TGFβ for 48 h. GAPDH and lamin were used as loading and fractionation controls for the cytoplasmic and nuclear fractions, respectively. Statistical analyses were performed using two-tailed t tests on at least three independent experiments. D, n = 5; E, n = 3. (F) BrdU (green) labeling and overlay of BrdU and ser780pRb (red) immunostaining in rat Schwann cells treated with TGFβ for 48 h (double stain appears yellow). (G) Images of single confocal sections of coimmunostaining of Ski (green) or ser780pRb (pRb; green) with EEA1 (early endosome marker; red) or P4D1 (ubiquitin; red) in rat Schwann cells treated with TGFβ for 48 h. Arrows indicate examples of the colocalization (appears yellow) of Ski or ser780pRb with EEA1 or P4D1. Insets are magnifications of the regions outlined by boxes. Error bars represent SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether proliferation of Schwann cells was correlated with the marked relocalization of ser780pRb into the cytoplasm, we double stained TGFβ-treated cells to detect ser780pRb and the incorporation of BrdU simultaneously. All BrDU-positive cells also showed a pronounced ser780pRb cytoplasmic staining (Fig. 3 F). Thus, we concluded that the relocalization of ser780pRb occurred in Schwann cells entering S phase. Interestingly, cytoplasmic Ski and Rb or ser780pRb exhibited a peculiar punctuate-like pattern. Some of these structures were labeled with the early endosomal marker EEA1 and the P4D1 antiubiquitin antibody (Fig. 3 G). However, the biological relevance of these findings remains to be determined. Each experiment was performed at least three times independently.
Upon TGFβ treatment, Ski and Rb colocalize and interact in the cytoplasm of Schwann cells but not of WB-F344 cells
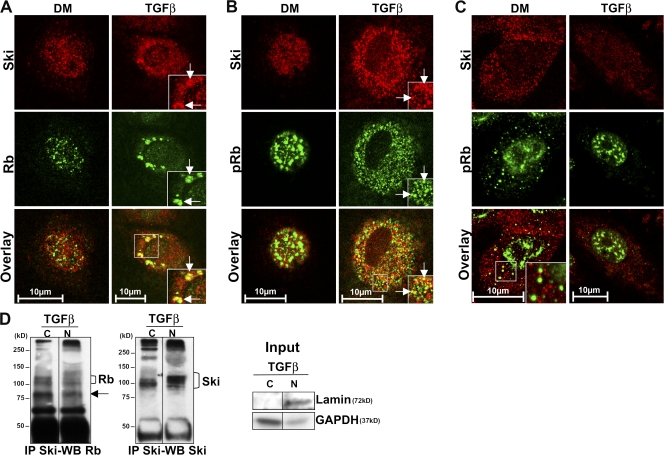
Because we had observed extensive relocalization of total Rb, ser780pRb, and Ski in TGFβ-treated Schwann cells cultured in DM, we next examined to what extent these proteins were colocalizing and potentially interacting under these conditions. Indeed, we found partial but pronounced colocalization of Rb and ser780pRb with Ski in the cytosol (Fig. 4, A, and B). In contrast to Schwann cells, we detected no colocalization of Ski with ser780pRb in WB-F344 epithelial cells (Fig. 4 C).
Figure 4.
TGFβ promotes Ski and Rb localization as a complex in the cytoplasm of Schwann cells but not of epithelial cells. (A–C) Images of single confocal sections of the coimmunostaining of Ski (red) and Rb (green; A) or Ski (red) and ser780pRb (pRb; green; B) in rat Schwann cells or in WB-F344 cells (C) cultured in DM or treated with TGFβ (the overlay appears yellow). Arrows indicate examples of the colocalization of Ski and Rb (A) and Ski and ser780pRb (B). Insets are magnifications of the regions outlined by boxes. (D) Immunoprecipitation of Ski and Western blotting of Rb or Ski in the cytoplasmic (C) and nuclear (N) fractions of rat Schwann cells treated with TGFβ for 36 h, and Western blot of lamin (nuclear marker) and GAPDH (cytoplasmic marker) performed on lysates used for immunoprecipitation (input). The arrow indicates a nonspecific band or a degradation product.
To test for physical interactions of the colocalized proteins, we performed subcellular fractionation of TGFβ-treated Schwann cell lysates followed by coimmunoprecipitation of Ski and total Rb. These techniques allowed us to show that Ski and Rb interact both in the nuclear and in the cytoplasmic compartments of Schwann cells treated with TGFβ (Fig. 4 D). Each experiment was performed at least three times independently.
After TGFβ treatment, the levels of Ski expression were strongly reduced in WB-F344 cells (Fig. 2 B), and no coimmunoprecipitation of Ski and Rb was detected using either the nuclear or the cytoplasmic fraction (unpublished data).
Ski and ser780pRb are mainly present and interact in the cytoplasm of Schwann cells in developing sciatic nerves, whereas low levels of nuclear ser780pRb are found in myelinating Schwann cells
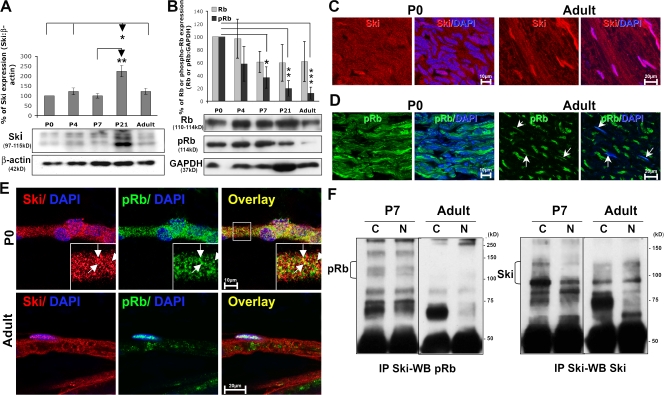
Our next aim was to relate our cell culture data to the in vivo situation. To approach this issue systematically, we started with quantitative assessments of Ski and Rb expression in developing rat sciatic nerves. Our Western blot analysis shows that Ski protein is present at every stage of postnatal development from postnatal day (P) 0 to adult in rat sciatic nerves, with a transient peak at P21 (Fig. 5 A). This peak of expression was surprising, and we therefore also measured the expression levels of Ski in mouse sciatic nerves. Here, Ski expression was not increased at P21 (unpublished data), leaving us with the only explanation of species-specific differences of unknown functional relevance. We then measured total Rb levels. Total Rb was expressed at all developmental time points and in the adult at comparable levels. In contrast, ser780pRb levels were high at early stages of postnatal development (from P0–4) when rat Schwann cells proliferate (Stewart et al., 1993) and decreased progressively until the adult stage (Fig. 5 B). Immunohistochemical stainings revealed that at P0, both Ski and ser780pRb are present and partially colocalize in both the cytoplasm and the nucleus, but the majority of each protein is localized in the Schwann cell cytoplasm. In adult rat sciatic nerves, however, Ski staining was equal in Schwann cell nuclei and cytoplasm (Fig. 5, C and E). The levels of ser780pRb in adult Schwann cells were generally low, and the residual expression was mostly restricted to the nucleus (Fig. 5, D and E). In the adult rat sciatic nerves, we detected some signal, presumably in axons (Fig. 5 D), which is likely to be nonspecific if the low expression of ser780pRb at this stage is considered. As anticipated from our in vitro results and expression data, we were able to coimmunoprecipitate Ski and ser780pRb from both nuclear and cytoplasmic fractions of P7 rat sciatic nerves, but not at the adult stage (Fig. 5 F). Each experiment was performed at least three times independently, and statistical analyses were performed using two-tailed t tests on three groups of animals for each age. We conclude that Ski and ser780pRb interact in developing Schwann cells but not in fully differentiated cells in the adult.
Figure 5.
In vivo, Ski and ser780pRb are found in the cytoplasm of developing Schwann cells, where they interact, whereas ser780pRb is mostly restricted to the nucleus of myelinating Schwann cells. (A and B) Western blot analysis of Ski (A) and Rb and ser780pRb (pRb; B) expression in developing rat sciatic nerves, and graphs representing the densitometry of the bands normalized to the loading control β-actin or GAPDH. Statistical analyses were performed using two-tailed t tests on three groups of animals for each age. (C and D) Images of single confocal sections of Ski (red; C) or ser780pRb (pRb; green; D) and DAPI (blue) immunostainings on longitudinal sections of P0 and adult rat sciatic nerves. Arrows point out Schwann cell nuclei. (E) Images of single confocal sections of Ski (red) and ser780pRb (pRb; green) coimmunostainings and DAPI (blue) labeling on teased nerve fibers of P0 and adult rat sciatic nerves. In C–E, Ski appears pink, and pRb appears turquoise when nuclear. In E, the overlay of Ski and pRb appears yellow. Arrows indicate examples of the colocalization of Ski and ser780pRb. Insets are magnifications of the regions outlined by boxes. (F) Immunoprecipitation of Ski and Western blot of ser780pRb (pRb) or Ski in the cytoplasmic (C) and nuclear (N) fractions of P7 and adult rat sciatic nerve lysates. Error bars represent SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
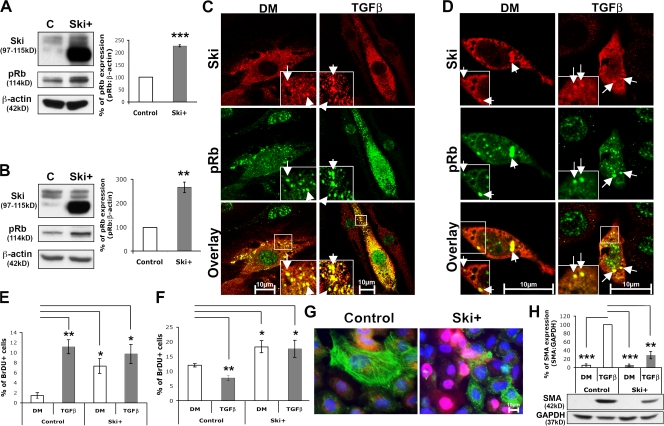
Ski overexpression in Schwann cells and WB-F344 cells leads to increased phosphorylation of Rb, TGFβ-independent cytoplasmic colocalization of Ski with ser780pRb, and increased cell proliferation
To achieve efficient exogenous overexpression of Ski, we used both adenoviral and lentiviral expression systems. Each overexpression experiment was performed at least three times with one viral vector, and the results were verified with the other viral vector. Ski overexpression mediated by these vectors led to significantly increased ser780pRb levels in growing medium (determined by Western blot analysis) in Schwann cells (Fig. 6 A) and WB-F344 cells (Fig. 6 B) and also in DM alone and in cells treated with TGFβ (determined by immunostaining; not depicted). Immunocytochemical analysis revealed that in both cell types, Ski and ser780pRb were strongly colocalized in the cytoplasm of Ski-overexpressing cells independently of TGFβ treatment (Fig. 6, C and D). Additionally, in both Schwann cells and WB-F344 cells overexpressing Ski, S-phase entry (assessed by BrdU incorporation) was increased independently of TGFβ treatment (Fig. 6, E and F). Interestingly, TGFβ-induced SMA expression was strongly reduced in Ski-overexpressing WB-F344 cells (Fig. 6, G and H), but the myelin proteins P0, PMP22, and periaxin were not significantly affected by Ski overexpression in Schwann cells (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200710161/DC1). Each experiment was performed at least three times independently, and statistical analyses were performed using two-tailed t tests.
Figure 6.
Ski overexpression up-regulates ser780pRb in rat Schwann cells and in epithelial cells and promotes TGFβ-independent Ski and ser780pRb cytoplasmic colocalization and the proliferation of rat Schwann cells and epithelial cells. (A and B) Western blot analysis of Ski and ser780pRb (pRb) in rat Schwann cells (A) and WB-F344 cells (B) kept in growing medium and infected with a control or a Ski-overexpressing (Ski+) lentivirus. (C and D) Images of single confocal sections of Ski (red) and ser780pRb (pRb; green) coimmunostaining in rat Schwann cells (C) and WB-F344 cells (D) both infected with a Ski+ adenovirus and cultured in DM or treated with TGFβ. Arrows indicate examples of the colocalization (appears yellow) of Ski and ser780pRb. Insets are magnifications of the regions outlined by boxes. (E and F) Percentage of BrdU-labeled rat Schwann cells (E) and WB-F344 cells (F) infected with a control or a Ski+ lentivirus and cultured in DM or treated with TGFβ. (G) SMA (green) and Ski (red) coimmunostaining and DAPI labeling (blue) in TGFβ-treated WB-F344 cells infected with a control or a Ski+ lentivirus. (H) Western blot analysis of SMA in lysates of WB-F344 cells infected with a control or a Ski+ lentivirus and cultured in DM or treated with TGFβ. (A, B, and H) β-Actin of GAPDH was used as loading control. Statistical analyses were performed using two-tailed t tests on at least three independent experiments. Error bars represent SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We conclude that the elevated expression of Ski promotes hyperphosphorylation of Rb, cell type– and TGFβ-independent cytoplasmic colocalization with ser780pRb, as well as cell proliferation.
Ski down-regulation in Schwann cells prevents TGFβ-induced proliferation and ser780pRb relocalization to the cytoplasm
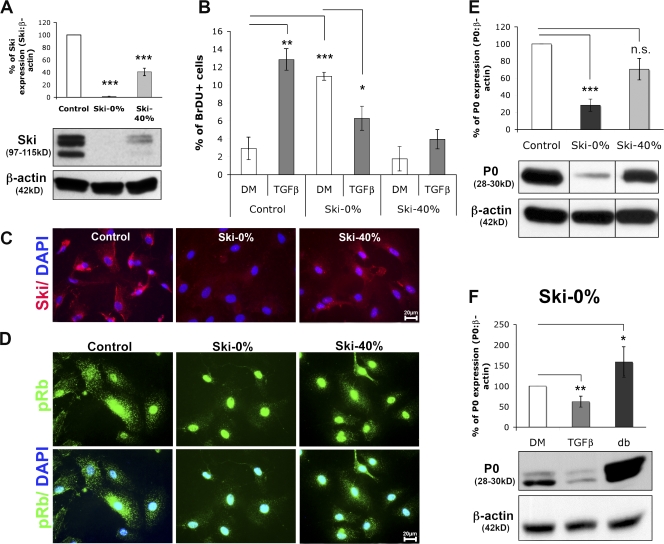
To perform loss-of-function experiments, we transduced Schwann cells with a lentivirus carrying a puromycin selection marker and a Ski-specific short hairpin RNA (shRNA) that was able to down-regulate endogenous Ski to either nondetectable levels (Ski-0%) or to 40% of its endogenous expression (Ski-40%) in puromycin-selected Schwann cells (Fig. 7, A and C). Concerning Ski-0%, all experiments were performed with two different Ski shRNAs with comparable efficiencies for down-regulation, yielding similar results in our assays. Ski levels of expression were quantified in Schwann cells cultured in growing medium and selected with puromycin.
Figure 7.
Ski down-regulation in Schwann cells prevents TGFβ-induced proliferation and ser780pRb localization in the cytoplasm. (A and C) Western blot analysis of Ski expression (A) and immunostaining of Ski (red) and DAPI (blue) labeling in Schwann cells infected with a control shRNA (control) or a Ski-specific shRNA (Ski-0% or Ski-40%) lentivirus and kept in growing medium (for Western blot analysis) or treated with TGFβ (for immunostaining; C). (B) Graph representing the percentage of BrdU-labeled rat Schwann cells infected with a control shRNA or a Ski-specific shRNA (Ski-0% or Ski-40%) lentivirus and cultured in DM or treated with TGFβ for 24 h. (D) Immunostaining of ser780pRb (pRb; green) and DAPI (blue) labeling (pRb appears turquoise when nuclear) in rat Schwann cells infected with a control or a Ski-specific shRNA (Ski-0% or Ski-40%) lentivirus and treated with TGFβ for 24 h. (E) Western blot analysis of P0 in rat Schwann cells infected with a control or a Ski-specific shRNA (Ski-0% or Ski-40%) lentivirus and cultured in DM. Lysates of cells infected with the control shRNA and the Ski-specific shRNA lentiviruses have been run on the same gel but not on consecutive lanes. (F) Western blot analysis of P0 in rat Schwann cells infected with a Ski-specific shRNA (Ski-0%) and cultured in DM or treated with TGFβ or dbCAMP for 24 h. For A, E, and F, β-actin was used as loading control, and the graphs represent the densitometry of the bands of the protein of interest normalized to the loading control. Statistical analyses were performed using two-tailed t tests on at least three independent experiments. Error bars represent SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Schwann cells expressing endogenous levels of Ski are growth arrested when cultured in DM, and their proliferation is stimulated upon TGFβ treatment. If 60% of endogenous Ski was knocked down (Ski-40%), Schwann cells were growth arrested in DM, and TGFβ did not significantly promote proliferation. Interestingly, if Ski expression was knocked down to undetectable levels in DM-cultured Schwann cells, proliferation was increased, and TGFβ reduced this proliferation (Fig. 7 B). We interpret these data as suggesting that Ski is also involved in the control of non-TGFβ–mediated Schwann cell proliferation and that a low level of Ski expression is sufficient for this regulation. If we consider the effect triggered by TGFβ on DM-cultured Schwann cells, TGFβ appears to be largely unable to stimulate their proliferation in the absence of Ski. Consistent with this notion, Ski knockdown blocked ser780pRb relocalization to the Schwann cell cytoplasm in the presence of TGFβ (Fig. 7 D). In DM, no cytoplasmic localization of ser780pRb was observed in either control cells or when Ski was down-regulated (unpublished data). Additionally, neither total Rb nor ser780pRb levels were affected by Ski down-regulation in growing medium (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200710161/DC1).
We next examined the influence of Ski knockdown on Schwann cell differentiation using the marker protein P0. Although the addition of TGFβ resulted in a decrease of proliferation when Ski levels were reduced completely (Fig. 7 B), this effect did not lead to an increase of P0 but rather resulted in the reduced expression of P0 under these conditions (Fig. 7 F). In DM, knockdown of Ski led to a decrease of P0 levels (Fig. 7 E) and an increase in proliferation (Fig. 7 B). However, upon dbcAMP treatment, P0 was up-regulated in the absence of Ski (Fig. 7 F). Each experiment was performed at least three times independently, and statistical analyses were performed using two-tailed t tests. These data indicate that dbcAMP can up-regulate P0 and that TGFβ can down-regulate P0 in the absence of Ski.
Discussion
The aim of this study was to elucidate the critical mechanisms by which TGFβ induces cell type–specific effects on the cell cycle. TGFβ can trigger Schwann cell proliferation or apoptosis, depending on culture conditions and maturation stage, whereas it mediates epithelial cell growth arrest and differentiation. In our culture conditions, TGFβ induced Schwann cell proliferation. Furthermore, TGFβ mediated the growth arrest of WB-F344 rat epithelial cells and promoted their differentiation, as indicated by size increase and SMA expression used as differentiation markers. Schwann cells are a key lineage in nerve development, supporting axonal growth and providing myelination, and TGFβ is an important regulator of Schwann cell proliferation during perinatal development (D'Antonio et al., 2006). The reasons why TGFβ promotes Schwann cell proliferation but induces growth arrest and differentiation of other cell types such as epithelial cells are largely unknown. In this study, we identify and describe a novel pathway induced by TGFβ in Schwann cells involving Ski and Rb and regulated by Ski levels of expression. The different regulation of this pathway in Schwann cells and epithelial cells is likely to be a major determinant of the observed differences in the outcome of TGFβ signaling in Schwann cells compared with epithelial cells.
In a previous study, we have found that Ski is involved in the control of Schwann cell proliferation and myelination (Atanasoski et al., 2004). The protooncogene Ski is a negative regulator of TGFβ-induced Smad2/3 activation and can interact with several different partners (Luo, 2004). Thus, to identify TGFβ-regulated pathways differing between Schwann cells and epithelial cells, we investigated the expression of described partners of Ski in cultured Schwann cells and epithelial cells. Rb was up-regulated in TGFβ-treated Schwann cells, whereas it remained unaltered in epithelial cells. Furthermore, TGFβ increased ser780pRb levels in Schwann cells but strongly decreased ser780pRb in epithelial cells, which, in principle, is consistent with the observed increase in proliferation of TGFβ-treated Schwann cells and the growth-arrested and differentiated state of TGFβ-treated epithelial cells. However, the up-regulation of both total Rb and ser780pRb in TGFβ-treated Schwann cells was peculiar. The classical definition of Rb is that of a negative regulator of the G1/S-phase transition. This function of Rb is achieved through its hypophosphorylated form by binding and inactivation of the transcription factor E2F. The hyperphosphorylated form of Rb is thought to be functionally inactive. Thus, the unusual Rb regulation in Schwann cells prompted us to follow up on this issue.
We found that after treatment with TGFβ, ser780pRb started to be relocalized to the cytoplasm of Schwann cells within 6 h, and relocalization was maximal after 48 h. This finding was surprising because Rb has been described as a nuclear protein. In the glioblastoma cell line T98G, however, serum induces cytoplasmic localization of the three pocket proteins p107, p130, and Rb and the nucleocytoplasmic shuttling of p130 (Chestukhin et al., 2002). It has been speculated that the pocket family members accumulate in the cytoplasm during the late G1 phase of the cell cycle to provide a rapid and efficient way to relieve pocket protein–mediated repression of E2F-dependent transcription. Additionally, in transformed human lung fibroblasts in which a Cdk4 mutation leads to loss of sensitivity to p16 inhibition (Jiao et al., 2006), hyperphosphorylated Rb is increased and mislocalized to the cytoplasm. This is possibly one of the reasons for allowing these transformed cells to evade growth-regulatory constraints. These previous studies used tumor cells that have acquired various mechanisms allowing escape from cell cycle exit (Chestukhin et al., 2002; Jiao et al., 2006). Importantly, we show here that cytoplasmic relocalization of Rb also occurs in primary Schwann cells during proliferation and is likely an important component of the normal regulatory circuit in this cell type. If Schwann cells were treated with TGFβ, Ski and ser780pRb moved in concert to the Schwann cell cytoplasm where Ski and ser780pRb partially colocalized and interacted. This is likely to be of physiological importance also in vivo because a similar cytoplasmic colocalization and interaction of Ski with ser780pRb was observed in early postnatal development of the sciatic nerve when Schwann cells still proliferate. Whether hyperphosphorylated Rb plays an active role in the regulation of cytoplasmic Ski (for example, by regulating its stability) remains to be determined.
Overexpression of Ski induced Rb hyperphosphorylation and the colocalization of both proteins in the cytoplasm of Schwann cells and epithelial cells. Furthermore, proliferation of Schwann cells and epithelial cells was stimulated independently of TGFβ, and TGFβ was unable to trigger efficient growth arrest and differentiation of Ski-overexpressing epithelial cells. These results indicate that a high expression of Ski promotes proliferation and suggest that at least a part of this effect is caused by the increased phosphorylation of Rb and its sequestration in the cytoplasm. These findings are in partial disagreement with our previous study (Atanasoski et al., 2004) in which Ski overexpression did not promote proliferation of Schwann cells in DM but decreased TGFβ-induced proliferation and up-regulated myelin gene transcripts and the myelin protein periaxin. We have not been able to identify the causes for these differences. We must assume that they are caused by different culture conditions or unknown biological variants. Different fetal calf sera, pituitary extracts, or preparation variability of primary Schwann cells are potential candidates.
When Ski was knocked down in Schwann cells, TGFβ was no longer able to stimulate proliferation compared with the DM condition, and there was no or very reduced phospho-Rb (pRb) cytoplasmic localization. When Ski was knocked down to undetectable levels, TGFβ even decreased proliferation. These results indicate that Ski expression modulates the effect of TGFβ on Schwann cell proliferation and pRb relocalization to the cytoplasm and suggest that pRb cytoplasmic localization is very likely to account for at least a part of TGFβ-induced proliferation of Schwann cells.
Our results further indicate that Ski is also involved in the control of other pathways than those induced by TGFβ because stimulated proliferation and a decrease of the myelin protein P0 were observed when Ski expression was knocked down to undetectable levels in Schwann cells cultured in DM only. These results are in agreement with our previous data showing reduced levels of myelin gene expression in Ski-deficient peripheral nerves and the lack of myelin in cultured dorsal root ganglia in the absence of Ski (Atanasoski et al., 2004).
In our present settings, dbcAMP was able to up-regulate the Schwann cell differentiation marker P0 in the absence of Ski, and overexpression of Ski did not affect the expression of P0 and other differentiation markers. These data suggest that culture conditions modulate Ski effects on Schwann cell differentiation. It is possible, although it remains to be demonstrated, that Ski does not act primarily on Schwann cell differentiation. The decrease of myelin proteins in DM in the absence of Ski could be the result of increased proliferation. In the present study, we show that Ski regulates proliferation depending on its level of expression and its subcellular localization. However, we cannot exclude that Ski is also directly involved in the control of Schwann cell differentiation under certain conditions. Importantly, proliferation and differentiation were not always interdependent in Schwann cells because we observed that proliferation was induced in Ski-overexpressing Schwann cells without affecting the expression of differentiation markers. In this study, we have focused our investigation on the function of Ski in the regulation of the TGFβ pathway. However, Ski seems to also regulate pathways that are not induced by TGFβ, and further work is necessary to fully understand the functions of Ski in Schwann cell biology.
In addition to the multiple effects of TGFβ on the cell cycle, proliferation, and differentiation addressed in this study, TGFβ also mediates Schwann cell apoptosis (Parkinson et al., 2004; D'Antonio et al., 2006). In fact, the balance of TGFβ-mediated control of Schwann cell proliferation and apoptosis has been elegantly documented in TGFβ receptor II–null mice (D'Antonio et al., 2006). Elucidating the molecular basis of the pathways mediating the apoptosis effects and the potential relationship to the mechanisms described here will be important topics for further studies. On a broader scale, a detailed understanding of the mechanisms controlling Schwann cell proliferation and survival are of wider significance to provide the basis to develop treatment of peripheral nerve tumors (Schwann cell hyperplasia), inherited peripheral neuropathies, and common peripheral neuropathies secondary to diabetes, cancer chemotherapeutic agents, toxins, and autoimmune disorders. Schwann cells are also under evaluation in transplantation paradigms to augment regeneration, when accident-caused large gaps in peripheral nerves have occurred, and as auxiliary cells in nonregenerating central nervous system lesions (e.g., spinal cord repair). Profound knowledge of the control of Schwann cell proliferation and differentiation is of key importance for the success of such applications in regenerative medicine.
With regard to epithelial cells, TGFβ signaling plays a critical role in the control of epithelial tumor formation as a tumor suppressor. We show here that if Ski levels are high, there is a concomitant increase in ser780pRb, proliferation is stimulated, and TGFβ is unable to promote growth arrest and differentiation. It is interesting to note in this context that Ski levels are up-regulated in many tumor cells (Nomura et al., 1989; Fumagalli et al., 1993). Up-regulation of Ski and its interaction with Rb in the cytoplasm may therefore constitute a mechanism by which cancer cells are able to escape from cell cycle exit.
Materials and methods
Cell culture
Primary Schwann cells were derived from P2–3 Wistar rat sciatic nerves and dissociated in 0.3 mg/ml collagenase type I (Sigma-Aldrich) and 2.5 mg/ml trypsin (Sigma-Aldrich) in DME (Invitrogen) at 37°C and 5% CO2/95% air for 1 h.
After the addition of DME containing 10% FCS (Invitrogen), cells were centrifuged at 500 g for 10 min, resuspended in DME containing 10% FCS, 1:500 penicillin/streptomycin (Invitrogen), and 10 μM cytosine arabinoside (Sigma-Aldrich), and plated on plastic dishes coated with poly-l-lysine (Sigma-Aldrich). After 24 h at 37°C and 5% CO2/95% air, cells were washed and incubated in Schwann cell growing medium (DME containing 10% FCS, 1:500 penicillin/streptomycin, 4 μg/ml of crude glial growth factor [bovine pituitary extract; BioReba Biotechnology, Inc.], and 2 μM forskolin [Sigma-Aldrich]) until they reached confluency. They were then purified by sequential immunopanning as described previously (Dong et al., 1997).
For growth arrest, cells were incubated for 2.5 d in DM containing 0.5% FCS, 1:500 penicillin/streptomycin, 100 μg/ml of human apotransferrin, 60 ng/ml progesterone, 1 μg/ml insulin, 16 μg/ml putrescine, 400 ng/ml l-thyroxin, 160 ng/ml selenium, 10 ng/ml triiodothyronine, and 300 μg/ml BSA in DME/F12 (Invitrogen). Supplements were purchased from Sigma-Aldrich. For treatment with 10 ng/ml TGFβ1 (R&D Systems) or 1 mM dbcAMP (Sigma-Aldrich), cells were incubated in DM overnight and treated for 2 d (unless stated differently). The rat epithelial cell line WB-F344 was provided by J.E. Trosko (Michigan State University, East Lansing, MI). WB-F344 cells were cultured in DME containing 10% FCS and 1:500 penicillin/streptomycin (growing medium). For differentiation, cells were incubated in DM overnight and treated with 10 ng/ml TGFβ1 for 4–5 d.
Preparation of cryosections and teased fibers
Animal use (Wistar and Sprague-Dawley rats; Elevage Janvier) was approved by the veterinary office of the Canton of Zurich, Switzerland. Processing of rat sciatic nerves was performed as previously described (Atanasoski et al., 2001).
Generation of adeno- and lentiviruses
The Ski-overexpressing adenovirus and its control were generated as described previously (Atanasoski et al., 2004). For the Ski-overexpressing lentivirus construct, the GFP sequence of the pLentiLox 3.7 construct (American Type Culture Collection) was excised, and the human Ski cDNA coding sequence was inserted between NheI and EcoRI restriction sites (EcoRI was blunted before insertion). The pLentiLox 3.7 construct expressing GFP was used as a control. Both Ski-0% shRNA constructs, the Ski-40% shRNA construct, and the Non-Target shRNA Control construct were purchased from Sigma-Aldrich. To produce lentiviral particles, HEK293T cells were cotransfected with each lentiviral construct together with the packaging constructs pLP1, pLP2, and pLP/vesicular stomatitis virus glycoprotein (Invitrogen) using Lipofectamine 2000 (Invitrogen) according to the recommendations of the manufacturer (ViraPower Lentiviral Expression Systems manual).
Infection of Schwann cells and WB-F344 cells with adenoviruses or lentiviruses
The Ski-overexpressing adenovirus was used as previously described (Atanasoski et al., 2004). In brief, adenoviral particles were added to rat Schwann cells in their growing medium and to WB-F344 cells in DM at an MOI of 1,000. The next day, cells were washed. Schwann cells were maintained in their growing medium for an additional 2 d before use for experiments, and WB-F344 cells were maintained in DM alone or treated with TGFβ for 4–5 d.
The Ski-overexpressing lentivirus or Ski shRNA lentiviruses were incubated overnight with Schwann cells or WB-F344 cells in their respective growing medium containing 8 μg/ml Polybrene (Sigma-Aldrich) at an MOI of 5. The next morning, cells were washed and maintained in their respective growing medium for an additional day. Cells transduced with Ski-overexpressing lentiviruses were then used for experiments. Schwann cells transduced with Ski shRNA lentiviruses were selected with 2 μg/ml puromycin for 2 d before use for experiments.
Western blotting
Sciatic nerves were dissected, frozen in liquid nitrogen, pulverized with a chilled mortar and pestle, lysed in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris/HCl, pH 7.4, 150 mM NaCl, 50 mM NaF, 1 mM NaVO4, 1 mM EDTA, 0.5% wt/vol sodium deoxycholate, and 1% Nonidet P-40) for 15 min on ice, and centrifuged to pellet debris. Supernatants were collected, and protein concentration was determined by bicinchoninic acid (BCA) assay (Bio-Rad Laboratories).
Cells were washed three times in PBS, lysed in RIPA buffer for 15 min on ice, and centrifuged to pellet debris. Sciatic nerves and cell lysates were submitted to SDS-PAGE and analyzed by Western blotting as described previously (Jacob et al., 2005).
The primary antibodies used were as follows: rabbit polyclonal anti-Ski (1:1,000; H-329; Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-Ski (1:1,000; Cascade Bioscience), mouse monoclonal anti-Rb (1:2,000; Chemicon), rabbit polyclonal anti-Rb (1:1,000; NeoMarkers), rabbit polyclonal anti-ser780pRb (1:2,000; Cell Signaling Technology), mouse monoclonal anti-P0 (1:1,000; Astexx Ltd), rabbit polyclonal antiperiaxin (1:1,000; provided by P. Brophy, University of Edinburgh, Edinburgh, Scotland, UK), rabbit polyclonal anti-PMP22 (homemade; 1:2,000), mouse monoclonal anti–β-actin (1:5,000; Sigma-Aldrich), mouse monoclonal anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:3,000; Hytest Ltd.), goat polyclonal antilamin (1:2,000; lamin A [C20]; Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti–cyclin D1 (1:2,000; C-20; Santa Cruz Biotechnology, Inc.), and mouse monoclonal anti-SMA (1:1,000; Sigma-Aldrich).
Immunoprecipitation
Sciatic nerves and cell lysates were prepared as described in the previous section. 1 ml of cleared lysates was rotated with immunoprecipitating antibodies (1 μg of rabbit polyclonal Ski) overnight at 4°C. 40 μl of protein A/G PLUS (Santa Cruz Biotechnology, Inc.) was added, and samples were rotated for 2 h at 4°C. Immunoprecipitates were pelleted, washed three times with RIPA buffer, boiled in laemmli buffer, and analyzed by Western blotting.
Subcellular fractionation
Cytoplasmic and nuclear fractions of sciatic nerves and cell lysates were separated as described previously (Wesemann et al., 2004) and analyzed by Western blotting or subjected to immunoprecipitation.
Densitometry
Blots were digitized using a scanner (ScanMaker X12 USL; Microtek) and analyzed by densitometry with Image 1.63 (National Institutes of Health).
Immunofluorescence
Cells were fixed with 4% PFA in 100 mM PBS, pH 7.4, for 20 min at 4°C, washed in PBS, blocked for 15 min in PBS containing 0.1% saponin (or 0.3% Triton X-100) and 2% goat serum, and incubated with primary antibodies (rabbit polyclonal Ski at 1:100; mouse monoclonal Ski at 1:100; mouse monoclonal Rb at 1:50; rabbit polyclonal ser780pRb at 1:400; mouse monoclonal SMA at 1:300; mouse monoclonal EEA-1 at 1:700 [Transduction Laboratories]; and mouse monoclonal ubiquitin [P4D1] at 1:200 [Santa Cruz Biotechnology, Inc.]) overnight at 4°C in blocking buffer. Cells were washed and incubated with secondary antibodies coupled to AlexaFluor488 or Cy3 for 1–2 h at room temperature (1:500–1:750; Jackson ImmunoResearch Laboratories).
For BrdU labeling assay, the reagents were obtained from BrdU Labeling and Detection kit I (Roche). In brief, cells were incubated with BrdU labeling reagent (1:1,000) for 1 h at 37°C with 5% CO2/95% air, washed with PBS, fixed with 70% ethanol in 50 mM glycine, pH 2.0, for 20 min at −20°C, washed, and incubated with anti-BrdU with nucleases (mouse monoclonal BrdU antibody; 1:25) in incubation buffer (provided in the kit) for 30 min at 37°C. Cells were washed in PBS and incubated with anti–mouse Ig-fluorescein (secondary antibody; 1:20) for 30 min at 37°C. When double labeling was performed, the other primary antibody was incubated at the same time as the BrdU antibody.
Cells were observed using a fluorescence microscope (Axioplan2 Imaging; Carl Zeiss, Inc.) with 20× 0.50 NA, 40× 0.75 NA, or 63× 1.25 NA oil immersion plan Neofluar objectives (Carl Zeiss, Inc.). Images were digitized with a camera (PowerShot G5; Canon) and acquired with Axiovision 4.5 software (Carl Zeiss, Inc.). Brightness and contrast of images were adjusted using Photoshop 7.0 (Macintosh version; Adobe).
For confocal analyses, cells were observed using an inverse microscope (DMIRE2; Leica) and a point laser-scanning confocal microscope (SP2 AOBS; Leica) with a 63× 1.4 NA differential interference contrast oil HCX Plan-Apo objective (Leica). Optical sections were collected at 0.2-μm intervals using LCS software (Leica). Images were assembled, and the brightness and contrast of images were adjusted using Photoshop 7.0. Single optical sections are shown.
Statistical analyses
Statistical analyses were performed using two-tailed t tests (unless stated otherwise). P-values are assigned as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Online supplemental material
Fig. S1 shows the levels of expression of the myelin proteins P0, PMP22, and periaxin in Ski-overexpressing Schwann cells. Fig. S2 includes the levels of expression of total Rb and ser780pRb in Schwann cells in which Ski is down-regulated to undetectable levels (Ski-0%). Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200710161/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Ned Mantei for critical reading of the manuscript. We thank Dr. P.J. Brophy, E. Stavnezer, C. Colmenares, J.E. Trosko, D.B. Parkinson, R. Mirsky, and K.R. Jessen for providing various reagents.
This work was supported by funds granted to C. Jacob by the European Commission (Marie Curie Intra-European Fellowship FP6-2004-Mobility-5, proposal No 023124), to S. Atanasoski and U. Suter by the Swiss National Science Foundation, to U. Suter by the National Center of Competence in Research Neural Plasticity and Repair and the Swiss Foundation for Research on Muscle Diseases, and to S. Atanasoski by Swiss Life (Jubiläumsstiftung für Medizinische Forschung).
Abbreviations used in this paper: dbcAMP, dibutyryl cAMP; DM, defined medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PMP22, peripheral myelin protein 22; pRb, phospho-Rb; Rb, retinoblastoma protein; RIPA, radioimmunoprecipitation assay; shRNA, short hairpin RNA; SMA, α smooth muscle actin.
References
- Alexandrow, M.G., M. Kawabata, M. Aakre, and H.L. Moses. 1995. Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor β1. Proc. Natl. Acad. Sci. USA. 92:3239–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasoski, S., S. Shumas, C. Dickson, S.S. Scherer, and U. Suter. 2001. Differential cyclin D1 requirements of proliferating Schwann cells during development and after injury. Mol. Cell. Neurosci. 18:581–592. [DOI] [PubMed] [Google Scholar]
- Atanasoski, S., L. Notterpek, H.Y. Lee, F. Castagner, P. Young, M.U. Ehrengruber, D. Meijer, L. Sommer, E. Stavnezer, C. Colmenares, and U. Suter. 2004. The protooncogene Ski controls Schwann cell proliferation and myelination. Neuron. 43:499–511. [DOI] [PubMed] [Google Scholar]
- Chen, C.A., J.C. Hwang, J.Y. Guh, J.C. Tsai, and H.C. Chen. 2006. TGF-β1 and integrin synergistically facilitate the differentiation of rat podocytes by increasing alpha-smooth muscle actin expression. Transl. Res. 148:134–141. [DOI] [PubMed] [Google Scholar]
- Chestukhin, A., L. Litovchick, K. Rudich, and J.A. DeCaprio. 2002. Nucleocytoplasmic shuttling of p130/RBL2: novel regulatory mechanism. Mol. Cell. Biol. 22:453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriano, S.C., and Y.Q. Chen. 1998. Insensitivity to growth inhibition by TGF-β1 correlates with a lack of inhibition of the CDK2 activity in prostate carcinoma cells. Oncogene. 17:1549–1556. [DOI] [PubMed] [Google Scholar]
- Connell-Crowley, L., J.W. Harper, and D.W. Goodrich. 1997. Cyclin Dl/cdk4 regulates Retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell. 8:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antonio, M., A. Droggiti, M.L. Feltri, J. Roes, L. Wrabetz, R. Mirsky, and K.R. Jessen. 2006. TGF type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J. Neurosci. 26:8417–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Z., C. Dean, J.E. Walters, R. Mirsky, and K.R. Jessen. 1997. Response of Schwann cells to mitogens in vitro is determined by pre-exposure to serum, time in vitro, and developmental age. Glia. 20:219–230. [PubMed] [Google Scholar]
- Eccleston, P.A., K.R. Jessen, and R. Mirsky. 1989. Transforming growth factor-β and gamma-interferon have dual effects on growth of peripheral glia. J. Neurosci. Res. 24:524–530. [DOI] [PubMed] [Google Scholar]
- Fumagalli, S., L. Doneda, N. Nomura, and L. Larizza. 1993. Expression of the c-ski proto-oncogene in human melanoma cell lines. Melanoma Res. 3:23–27. [DOI] [PubMed] [Google Scholar]
- Hannon, G.J., and D. Beach. 1994. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 371:257–261. [DOI] [PubMed] [Google Scholar]
- Harrisingh, M.C., E. Perez-Nadales, D.B. Parkinson, D.S. Malcolm, A.W. Mudge, and A.C. Lloyd. 2004. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 23:3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, L.E., Y. Qian, and D.J. Templeton. 1995. G1 cyclins control the retinoblastoma gene product growth regulation activity via upstream mechanisms. Cell Growth Differ. 6:395–407. [PubMed] [Google Scholar]
- Iavarone, A., and J. Massague. 1999. E2F and histone deacetylase mediate transforming growth factor β repression of cdc25A during keratinocyte cell cycle arrest. Mol. Cell. Biol. 19:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, C., G.S. Cottrell, D. Gehringer, F. Schmidlin, E.F. Grady, and N.W. Bunnett. 2005. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J. Biol. Chem. 280:16076–16087. [DOI] [PubMed] [Google Scholar]
- Jiao, W., J. Datta, H.M. Lin, M. Dundr, and S.G. Rane. 2006. Nucleocytoplasmic shuttling of the Retinoblastoma tumor suppressor protein via cdk phosphorylation-dependent nuclear export. J. Biol. Chem. 281:38098–38108. [DOI] [PubMed] [Google Scholar]
- Li, J.M., P.P. Hu, X. Shen, Y. Yu, and X.F. Wang. 1997. E2F4-Rb and E2F4-p107 complexes suppress gene expression by transforming growth factor β through E2F binding sites. Proc. Natl. Acad. Sci. USA. 94:4948–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg, A.S., and R.A. Weinberg. 1998. Functional inactivation of the Retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell. Biol. 18:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, K. 2004. Ski and SnoN: negative regulators of TGF-β signaling. Curr. Opin. Genet. Dev. 14:65–70. [DOI] [PubMed] [Google Scholar]
- Mikaelian, I., M. Hovick, K.A. Silva, L.M. Burzenski, L.D. Shultz, C.L. Ackert-Bicknell, G.A. Cox, and J.P. Sundberg. 2006. Expression of terminal differentiation proteins defines stages of mouse mammary gland development. Vet. Pathol. 43:36–49. [DOI] [PubMed] [Google Scholar]
- Nomura, N., S. Sasamoto, S. Ishii, T. Date, M. Matsui, and R. Ishizaki. 1989. Isolation of human cDNA clones of ski and the ski-related gene, sno. Nucleic Acids Res. 17:5489–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, D.B., A. Bhaskaran, A. Droggiti, S. Dickinson, M. D'Antonio, R. Mirsky, and K.R. Jessen. 2004. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 164:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol, J.A., J.T. Holt, R.W. Stein, and H.L. Moses. 1990. Transforming growth factor β1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc. Natl. Acad. Sci. USA. 87:3758–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak, K., J.Y. Kato, M.J. Solomon, C.J. Sherr, J. Massague, J.M. Roberts, and A. Koff. 1994. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 8:9–22. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., J.B. Davis, P. Stroobant, and H. Land. 1989. Transforming growth factors-β 1 and β 2 are mitogens for rat Schwann cells. J. Cell Biol. 109:3419–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, M., D. Javelaud, and A. Mauviel. 2004. TGF-β-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 35:83–92. [DOI] [PubMed] [Google Scholar]
- Schwarz, J.K., C.H. Bassing, I. Kovesdi, M.B. Datto, M. Blazing, S. George, X.F. Wang, and J.R. Nevins. 1995. Expression of the E2F1 transcription factor overcomes type β transforming growth factor-mediated growth suppression. Proc. Natl. Acad. Sci. USA. 92:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, H.J., L. Morgan, K.R. Jessen, and R. Mirsky. 1993. Changes in DNA synthesis rate in the Schwann cell lineage in vivo are correlated with the precursor-Schwann cell transition and myelination. Eur. J. Neurosci. 5:1136–1144. [DOI] [PubMed] [Google Scholar]
- Sun, Y., X. Liu, E. Ng-Eaton, H.F. Lodish, and R.A. Weinberg. 1999. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor β signaling. Proc. Natl. Acad. Sci. USA. 96:12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokitou, F., T. Nomura, M.M. Khan, S.C. Kaul, R. Wadhwa, T. Yasukawa, I. Kohno, and S. Ishii. 1999. Viral ski inhibits retinoblastoma protein (Rb)-mediated transcriptional repression in a dominant negative fashion. J. Biol. Chem. 274:4485–4488. [DOI] [PubMed] [Google Scholar]
- Wesemann, D.R., Q. Hongwei, N. Kokorina, and E.N. Benveniste. 2004. TRADD interacts with STAT1-alpha and influences interferon-gamma signaling. Nat. Immunol. 5:199–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.