Abstract
Transfusional iron overload leads to gonadal failure and low bone mass in patients with thalassemia (Thal). However, gonadal failure is rarely reported in transfused patients with sickle cell disease (SCD) and the literature regarding fracture prevalence in SCD is limited. The objective of this study was to assess self-reported fracture prevalence and its relationship to endocrinopathy in transfused Thal or SCD subjects and compare to non-transfused subjects with SCD (NonTxSCD). Eligibility was based on age ≥12 years and liver iron concentration ≥ 10 mg/g dry wt or serum ferritin ≥ 2000 ng/mL (Thal or TxSCD) or for NonTxSCD, ferritin < 500 ng/mL. Data were collected by patient interview and chart review at 31 clinical centers in the U.S., Canada and the U.K. 152 subjects with Thal (52% Male; 25.6±0.7 yrs), 203 subjects with TxSCD (44% Male, 24.7 ±0.9 years: Mean ± SE), and 65 NonTxSCD (50% Male, 22.2 ±1.3 yrs) were enrolled. Overall, male subjects with Thal were more likely to have sustained a fracture in their lifetime (51%) compared to TxSCD (28%) or NonTxSCD (32%) (p=0.005). There was no difference in fracture prevalence among women (Thal: 26%, TxSCD 17%, NonTxSCD: 16%). Fracture was most frequently reported in the upper extremities (53.3% of all fractures) while spine and pelvic fractures were relatively common for such a young cohort: 10.6%. Though overall fracture prevalence was not distinctly different from published healthy cohorts, fewer fractures occurred during the adolescent years. In multivariate analysis, the significant predictors of fracture prevalence were Thal diagnosis (Odds Ratio: 2.3; 1.2–4.6; 95%CI), male gender (OR: 2.6; 1.5–4.5), hypothyroidism (OR: 3.3; 1.1–9.8) and age (OR: 1.1; 1.03–1.08). These data suggest that despite similar iron burden, transfused patients with Thal are at greater risk for fracture than subjects with SCD. Male subjects with Thal and hypothyroidism are at particular risk for fracture, in contrast, transfused subjects with SCD had no greater risk of fracture compared to non-transfused SCD. Though ethnic differences in fracture risk cannot be ignored, endocrinopathy is rare in TxSCD which may also provide some protection from fracture.
Keywords: Fracture, Endocrinopathy, Iron Overload, Thalassemia, Sickle Cell Disease
Introduction
Fracture incidence is well studied in healthy populations revealing a bi-modal curve, with peaks typically in the second and following the fourth decades of life [1]. Before 20 years of age, fracture is more common in males than females, primarily resulting from recreational activities [1,2,3]. Above 40 years of age, fracture is more common in females primarily the result of low bone mass [1,4]. Fracture in older patients results in increased morbidity, decreased independence and even elevated risk for mortality [5]. As patients with hematological disorders live longer, bone pain, osteoporosis and concomitant fracture are proving to be sources of significant morbidity [6,7,8,9].
Thalassemia and sickle cell anemia are the two most common hemoglobinopathies in North America, combined they affect over well over 70,000 individuals. Though distinct in presentation, both groups exhibit similar risk factors for low bone mass: delayed growth and pubertal development [10,11], milk avoidance, lactose intolerance, bone forming nutritional deficiencies (Calcium, Vit D, Zinc)[12,13,14], as well as depressed serum 25-OH vitamin D [15,16]. Additionally chronic hemolytic anemia or ineffective erythropoiesis leads to widening of medullary cavities and intratrabecular spaces and decreased cortical thickness [17,18]. Both groups also have a number of factors which may prove protective against fracture: severe anemia leads to decreased physical activity and fewer opportunities for recreational fractures [19]; patients with sickle cell disease who experience hypoxic episodes tend to have more pain and are therefore less apt to exercise; and parental overprotection may decrease exposure of the chronically ill child to fall risk.
Chronic transfusion is a life-saving therapy for many patients with Thal, whereas for SCD it is being used with increasing frequency to decrease risk for stroke. Though beneficial, transfusion therapy results in iron overload and iron-related organ toxicities in patients without strict adherence to chelation therapy. Although iron-related endocrine abnormalities are observed more frequently in Thal [20], the relationship of endocrinopathy to fracture prevalence has not been investigated in detail in either Thal or SCD.
A natural history, multi-center study was conducted to assess self-reported fracture history and iron-related endocrine dysfunction in transfused Thal and SCD subjects and compare to non-transfused subjects with SCD (NonTxSCD). The primary objective of this study was to determine whether fracture risk was similar in the 2 heavily iron overloaded groups. The secondary objectives were to compare fracture prevalence between iron overloaded and non-iron overloaded SCD subjects and to explore factors related to fracture in these unique patient populations.
Materials and Methods
The Multi-Center Study of Iron Overload was a prospective, longitudinal, natural history comparative study of organ dysfunction in adolescents and adults with transfusional iron overload and thalassemia or sickle cell disease and a control group of SCD subjects without iron overload. Subjects were recruited from 31 clinical hematological centers in the United States, Canada and the United Kingdom. The protocol and consenting documents were developed at the coordinating center (Children’s Hospital & Research Center, Oakland) and full institutional approval was obtained at each participating center. Informed written consent was obtained from all subjects and/or parents for subjects <18 years of age, and assent obtained from child participants in accordance with each institutions policy.
Subjects
Transfused subjects with sickle cell disease (Hb SS-type or Hb S β° Thalassemia) or thalassemia (β- or E-β-thalassemia) who were 12 years of age or older were screened at each center for eligibility. Eligibility was based on a liver iron concentration (LIC) of ≥ 10 mg/g dry weight by percutaneous biopsy within 2 years of screening or an average serum ferritin value within the previous 12 months of ≥ 2000 ng/dL. Non-transfused subjects with SCD (NonTx-SCD) were considered eligible if they were ≥ 12 years with an average serum ferritin value of < 500 ng/dL. Subjects with a history of genetic, metabolic, neuro-degenerative or other medical disease known to impact bone, endocrine, hepatic or cardiac organs were excluded.
Procedures
After enrollment, each subject’s medical history was reviewed by subject interview and medical record review as previously described [21]. The subject interview questionnaire included sections on demographics, medical, orthopedic and surgical history, recent hospitalizations, family history of endocrine complications, nutritional supplements, medications, tobacco and alcohol usage. A self-reported fracture history questionnaire included site of, age at, reason for and treatment of each fracture. Fracture etiology was organized into one of five categories: fall, sports/recreational injury, motor vehicle accident, heavy object or other/unknown. After review of the etiology of all fractures, those in the “other” category which were described as “fight” or “assault” were analyzed separately. Fracture treatment was organized into one of 4 categories: cast/splint, surgery, hospitalization or no treatment. If a specific treatment was given two treatment categories, such as requiring surgery and casting, the more serious treatment was chosen, e.g. surgery. After review of all fractures, those considered minor (e.g. finger, nose, toe fractures) or questionable were removed from the analysis prior to categorization. Nineteen minor fractures were sustained in all, suffered by 4.6% of Thal, 3.9% of TxSCD, and 1.5% of NonTxSCD. The remaining fractures were then divided into the following categories: upper extremity (shoulder, arm, forearm, wrist, or hand fracture), lower extremity (leg, ankle, foot), spine/back/pelvis, and other (rib, clavicle, collar bone, skull or undefined). Given site of fracture was by self report, the description of fracture location was frequently non-specific, e.g. lower leg vs. distal tibia. If further clarification was not made with a subject, the original description provided by the subject was unaltered.
Height and weight was obtained in all subjects and a validated, self-reported pubertal assessment was completed in those < 21 years [22]. The medical record review included documentation of transfusion and chelation history and recent laboratory values. Chronic transfusion was defined as ≥ 8 transfusions per year or 1 transfusion at least every 7 weeks. Endocrinopathy was described in detail previously [20], and was considered as dysfunction in at least one of four axis studied: growth failure, diabetes, hypogonadism or hypothyroidism. Growth failure was defined as a height Z-score of less than −2.5 and/or ongoing growth hormone therapy; diabetes was defined as a fasting glucose > 126 mg/dL and/or non-fasting glucose > 200 mg/dL and/or exogenous insulin administration or oral hypoglycemic medications; hypothyroidism was defined as ongoing thyroid hormone replacement therapy; and hypogonadims in females was defined as >13 yrs, not yet Tanner B2 or >14 yrs requiring estrogen replacement therapy or >15 yrs with primary amenorrhea, hypogonadism in males >14 yrs, not yet Tanner G2 or on androgen replacement therapy or >17 yrs, not yet Tanner G4. Though the adequacy of treatment was not specifically addressed, all subjects were under routine medical treatment due to their primary hematological condition.
Data analysis
Differences in continuous variables between groups (Thal vs. Tx-SCD, Tx-SCD vs. NonTx-SCD) at baseline were analyzed by Student’s t-tests. For categorical outcomes, differences between groups were analyzed using Pearson chi-square tests. Fisher’s exact tests were used for 2 by 2 comparisons. The ferritin data was skewed and therefore log-transformed before performing statistical tests. Descriptive data are presented as percentages or mean ± SD. The main outcomes of interest were: 1) fracture prevalence at baseline among groups, 2) fracture prevalence by age group, and 3) age of first fracture. Fracture prevalence was calculated as the percentage of all reported fractures at the baseline time point divided by the total number of subjects enrolled. Analysis of time to first fracture was performed using Kaplan-Meier survival estimates with age in years as the unit of analysis time. Period prevalence for each age group was calculated by dividing the number of fractures that occurred at a specific age (in years) by the total number of subjects for whom we had information at that age. Therefore, each subject contributes total person-years of follow-up equal to their age at baseline. Variables associated with fracture prevalence were determined using logistic regression techniques. Significance was defined at a p<0.05 level.
Results
203 iron-overloaded subjects with SCD, 152 subjects with Thalassemia and 65 non-iron overloaded subjects with SCD were eligible and completed baseline assessment (Table 1). Subject age and gender distribution were not different among groups (Table 1). By design, the transfused Thal and SCD subjects had significantly higher serum ferritin values compared to the NonTxSCD subjects. As previously reported [20] shorter transfusion duration and higher iron load were observed in the TxSCD subjects, yet the transfused Thal subjects had a much higher prevalence of endocrinopathy compared to TxSCD.
Table 1.
Demographics, Growth, Menarche and Iron Assessment Variables by Subject Diagnosis
| Variable | THAL (n=152) | Tx-SCD (n=203) | NonTx-SCD (n=65) | p-value (Thal vs. Tx- SCD) | p-value (TxSCD vs. NonTx- SCD) | |
|---|---|---|---|---|---|---|
| Age, years | Mean (SD) | 25.5 (8.1) | 24.7 (13.2) | 25.1 (11.4) | NS | NS |
| Subjects, > 18 yrs | N (%) | 118 (77.6) | 101 (49.8) | 41 (63.1) | <0.001 | NS |
| Male | N (%) | 80 (52.6) | 88 (43.4) | 33 (50.7) | NS | NS |
| Weight Z-score | Mean (SD) | −1.1 (1.4) | −0.3 (1.3) | −0.4 (1.2) | <0.001 | NS |
| Height Z-score | Mean (SD) | −1.6 (1.2) | −0.6 (1.3) | −0.1 (1.1) | <0.001 | 0.04 |
| Age at Menarche, y c | Mean (SD) | 14.3 (2.3) | 13.9 (2.2) | 14.9 (1.9) | NS | 0.05 |
| Serum Ferritin, ng/mL d | Mean (SD) | 3605 (2322) | 4338 (3013) | 108 (83) | <0.001 | <0.001 |
| Transfusion Duration, y | Mean (SD) | 21.3 (8.8) | 9.8 (7.2) | <0.001 | -- | |
| Range | (0.6–42.5) | (0.3–40.2) | --- | |||
| Any Endocrinopathy e | % | 85 (55.9) | 26 (12.8) | 5 (7.7) | <0.001 | NS |
For continuous variables, p-value for the difference between two groups is based on Student’s t-test. For categorical variables, p-value for the difference between groups is based on Pearson’s chi-square test.
BMI= Body Mass Index, kg/m2
Age at onset of menarche from only those females who successfully achieved menarche by the time of the baseline evaluation
Serum ferritin, is the average of 2 to 3 values analyzed within the previous 12 months. Mean values are provided in the table. Due to the skewness of these data, log transformed values were used in all analyses
Any endocrinopathy included evidence of diabetes, growth failure, hypogonadism or hypothyroidism.
Overall, subjects with Thal were more likely to have reported suffering a fracture in their lifetime than those with Tx-SCD or NonTx-SCD (Table 2, p<0.001). A closer analysis revealed that this difference was greatest between the male subjects (p=0.002), as there were no differences in fracture prevalence among women by diagnosis. Within the sub-group of subjects who had sustained a fracture, Thal subjects suffered their first fracture at an earlier age compared to TxSCD (p<0.001, Figure 1). Subjects with Thal also had a 5.6 fold greater odds of suffering multiple fractures, after adjusting for age, compared to Tx and NonTxSCD subjects (p=0.002). The majority of these multiple fractures were sustained in the upper extremities (23 arm, 13 wrist, 3 elbow) followed by the lower extremities (10 leg, 7 ankle, 4 foot). For SCD subjects with a history of fracture, NonTx subjects suffered more fractures than Tx subjects (p=0.02). Recurrent fractures, or those occurring at a similar site to a previous fracture, occurred in 38% of Thal, 29% of Tx-SCD and 40% of NonTx-SCD subjects with previous fracture. There was a strong inter-relationship between ethnicity and diagnosis, therefore the effect of ethnicity on fracture prevalence could be investigated in the Thal group only. No differences in fracture prevalence by ethnicity within the Thal group were observed.
Table 2.
Characteristics of Fractures in Subjects with Thalassemia, Transfused and Non-Transfused Sickle Cell Disease Subjects
| Characteristic, Unit | All Subjects (n=420) | Thal a (n=152) | Tx-SCD (n=203) | NonTx-SCD (n=65) | p-value (Thal vs. Tx-SCD) b | p-value (Tx-SCD vs. NonTx-SCD) |
|---|---|---|---|---|---|---|
| Fracture Prevalence, N (%) | 118 (28.1) | 59 (38.8) | 44 (21.7) | 15 (23.1) | <0.001 | NS |
| Females | 44 (20.1) | 19 (26.4) | 20 (17.4) | 5 (15.6) | NS | NS |
| Males | 74 (36.8) | 40 (50.0) | 24 (27.3) | 10 (30.3) | 0.002 | NS |
| No. Fracturesc, Mean±SD | 1.6 (1.1) | 1.9 (1.2) | 1.2 (0.5) | 1.9 (1.5) | 0.001 | 0.02 |
| Total number of fractures | 182 | 104 | 52 | 26 | ||
| Age at first fracture, Mean±SD | 13.7 (9.8) | 11.4 (8.1) | 17.2 (11.7) | 13.2 (7.5) | 0.01 | NS |
| Sites of Fracture | ||||||
| Upper Extremity | 98 (53.8) | 61 (58.7) | 23 (44.2) | 14 (53.8) | ||
| Lower Extremity | 53 (29.1) | 30 (28.8) | 19 (36.5) | 4 (15.4) | ||
| Spine, Back, Pelvis | 19 (10.4) | 10 (9.6) | 5 (9.6) | 4 (15.4) | ||
| Other/undefined | 12 (6.6) | 3 (2.9) | 5 (9.6) | 4 (15.4) | ||
| Cause of Fracture | ||||||
| Fall | 94 (51.6) | 59 (56.7) | 26 (50.0) | 9 (34.6) | ||
| Recreational | 43 (23.6) | 24 (23.1) | 10 (19.2) | 9 (34.6) | ||
| Motor Vehicle | 13 (7.1) | 9 (8.7) | 3 (5.8) | 1 (3.8) | ||
| Heavy Object | 6 (3.3) | 6 (5.8) | 0 (0.0) | 0 (0.0) | ||
| Other/Unknown | 26 (14.3) | 6 (5.8) | 13 (25.0) | 7 (26.9) | ||
| Treatment of Fracture | ||||||
| Cast/Splint | 144 (79.1) | 89 (85.6) | 38 (73.1) | 17 (65.4) | ||
| Surgery | 8 (4.4) | 3 (2.9) | 4 (7.7) | 1 (3.8) | ||
| Hospitalization | 6 (3.3) | 1 (1.0) | 1 (1.9) | 4 (15.4) | ||
| Not Treated | 14 (7.7) | 9 (8.7) | 3 (5.8) | 2 (7.7) | ||
| Unknown/not reported | 10 (5.5) | 2 (1.9) | 6 (11.5) | 2 (7.7) | ||
THAL: Thalassemic subjects; Tx-SCD: Transfused sickle cell subjects, NonTx-SCD subjects
p-values for differences between groups are based on Pearson’s chi-square tests for categorical variables or ANOVA for continuous variables. The p-value for age at splenectomy is determined using Kruskal-Wallis test. Superscripts denote differences between groups at p<0.05 using Pearson’s chi-square tests or Fisher’s exact tests for categorical variables and Mann-Whitney two-sample tests for age at splenectomy.
Average number of fractures in those who had fractured.
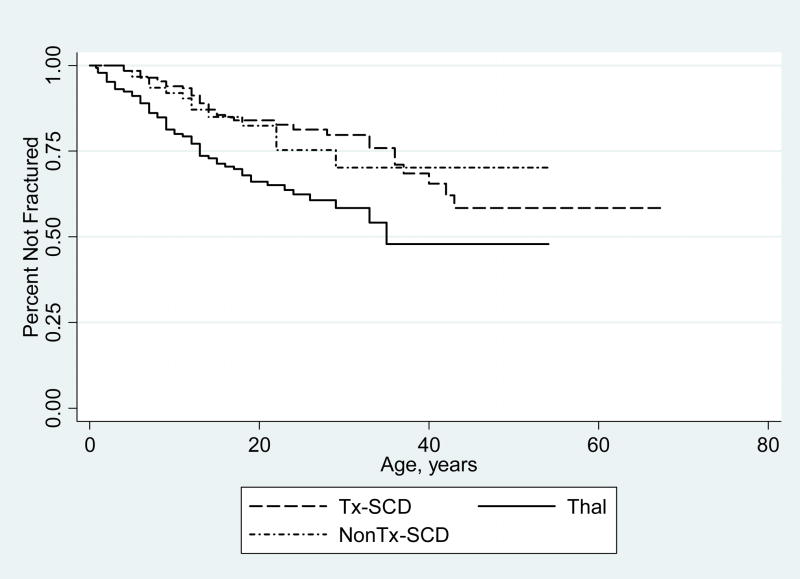
Figure 1.
Time to first fracture by diagnosis: Subjects with Thalassemia suffered their first fracture at an earlier age (11.4 yrs, solid line) compared to subjects with Tx-SCD (17.2 yrs, dashed line) or subjects with NonTx-SCD (13.2 yrs, dotted line).
When all three groups are combined, there was no difference in age at which fractures occur by gender. However, 31% of males had sustained a fracture by the age of 18, compared to only 17% of females (p=0.006). The most common site of fracture was the upper extremity (53.8% of all fractures, Table 2). Fractures which occur at the spine, hip and pelvis are typically rare except cases of significant trauma or in post-menopausal women who have significantly reduced bone mineral density. However, in this relatively young cohort of subjects, 19 subjects (4.5% of whole cohort) suffered these types of fractures. Of these, 3 resulted from significant trauma such as a motor vehicle accident or involvement with a heavy object, 9 were subsequent to a fall and 3 occurred as a result of a sporting event. Only four subjects suffered these types of fractures under the age of 21 in the absence of trauma or accident, all with a Thal diagnosis.
Additionally, in the group as a whole, there were a total of 5 fractures that resulted from a fight. These 5 fractures occurred in 1 Thal, 1 Tx-SCD and 2 NonTx-SCD subjects. There were an additional 3 fractures resulting from an assault, equally divided amongst subjects in the 3 diagnoses. If traumatic incidents are excluded from the analysis, that is fractures suffered from a motor vehicle accident, bike or bus accident, fight, assault, or heavy object, a more objective analysis can be made between the diagnoses. Therefore, when these remaining fractures are analyzed, 33.8% of the Thal subjects suffered atraumatic fractures compared to only 18% of the Tx-SCD (p=0.001). There were no significant differences in how fractures were treated among subject groups. As would be expected, over two thirds of all fractures were treated with a cast or splint, while close to 8% were treated with surgery or hospitalization (Table 2). Fractures treated with surgery or hospitalization (n=14) were sustained primarily from a fall (n=8). Surgical procedures were used to treat hip (n=3), arm (n=1), femur (n=1), knee (n=1) and jaw (n=1) fractures, whereas all of those treated by hospitalization were rib fractures. An additional 7% were not treated, these fractures mainly occurred in the rib (n=5), clavicle (n=1), hand (n=1), foot (n=2) and elbow (n=1).
Fracture prevalence was also compared by age group (Table 3). When adolescents (12–18 yrs) are analyzed separately, there was no difference in fracture prevalence by diagnosis, between 12.5 and 17.7% of subjects had sustained a fracture. Whereas, for adults (19+ yrs), Thal subjects have a higher fracture prevalence (44.9%) compared to TxSCD (26.7%, p=0.005); there was no difference in fracture prevalence in the adult TxSCD compared to the NonTxSCD group. Though there appeared to be differences in fracture prevalence by gender when separated by age group, given the smaller number of total fractures, these differences were not significant. Moreover, when fracture cause, etiology and treatment are compared amongst the adult vs. the adolescent subjects, no statistically significant trends were observed.
Table 3.
Characteristics of Fractures in Subjects with Thalassemia, Transfused and Non-Transfused Sickle Cell Disease Subjects by Age Group
| Characteristic, Unit | Thal a | Tx-SCD | NonTx-SCD | p-value (Thal vs. Tx-SCD) b | SCD vs. NonTx-SCD) |
|---|---|---|---|---|---|
| Adults, 19+ yrs | n=118 | n=101 | n=41 | ||
| Fracture Prevalence, N (%) | 53 (44.9) | 27 (26.7) | 12 (29.3) | 0.005 | NS |
| Females | 18 (34.0) | 13 (20.3) | 4 (20.0) | NS | NS |
| Males | 35 (53.9) | 14 (37.8) | 8 (38.1) | NS | NS |
| Total number of fractures | 95 | 34 | 21 | ||
| Sites of Fracture | |||||
| Upper Extremity | 53 (55.8) | 15 (44.1) | 11 (52.4) | ||
| Lower Extremity | 29 (30.5) | 12 (35.3) | 3 (14.3) | ||
| Spine, Back, Pelvis | 10 (10.5) | 5 (14.7) | 4 (19.1) | ||
| Other/undefined | 3 (3.2) | 2 (5.9) | 3 (14.3) | ||
| Cause of Fracture | |||||
| Fall | 54 (56.8) | 19 (55.9) | 6 (28.6) | ||
| Recreational | 20 (21.1) | 5 (14.7) | 8 (38.1) | ||
| MVA, Object, Other | 21 (22.1) | 10 (29.4) | 7 (33.3) | ||
| Adolescents, 12–18 yrs | n=34 | n=102 | n=24 | ||
| Fracture Prevalence, N (%) | 6 (17.7) | 17 (16.7) | 3 (12.5) | NS | NS |
| Females | 1 (5.3) | 7 (13.7) | 1 (8.3) | NS | NS |
| Males | 5 (33.3) | 10 (19.6) | 2 (16.7) | NS | NS |
| Total number of fractures | 9 | 18 | 5 | ||
| Sites of Fracture | |||||
| Upper Extremity | 8 (88.9) | 8 (44.4) | 3 (60.0) | ||
| Lower Extremity | 1 (11.1) | 7 (38.9) | 1 (20.0) | ||
| Other/undefined | 0 (0.0) | 3 (16.7) | 1 (20.0) | ||
| Cause of Fracture | |||||
| Fall | 5 (55.6) | 7 (38.9) | 3 (60.0) | ||
| Recreational | 4 (44.4) | 5 (27.8) | 1 (20.0) | ||
| MVA or Other | 0 (0.0) | 6 (33.3) | 1 (20.0) | ||
THAL: Thalassemic subjects; Tx-SCD: Transfused sickle cell subjects, NonTx-SCD subjects
p-values for differences between groups are based on Pearson’s chi-square tests for categorical variables or ANOVA for continuous variables. The p-value for age at splenectomy is determined using Kruskal-Wallis test. Superscripts denote differences between groups at p<0.05 using Pearson’s chi-square tests or Fisher’s exact tests for categorical variables and Mann-Whitney two-sample tests for age at splenectomy.
When the transfused subjects are analyzed together (Thal and TxSCD only, Table 4), there were no differences in height or weight Z-scores between those who fractured and those who did not fracture, and no greater likelihood of those who fractured to be taking growth hormone. There was also no difference in age of transfusion or chelation initiation; however those who fractured were transfused and chelated for a longer duration than those without history of fracture. There was also no relationship between liver iron concentration and fracture in the subset of subjects who had a recent biopsy (n=151). Those who fractured were more likely to have hypogonadism (p=0.005) and hypothyroidism (p=0.002).
Table 4.
Factors Related to Fracture Prevalence: Unadjusted relationships for Thal and Tx-SCD subjects only (n=351)
| Factor | Fracture (n=103) | No Fracture (n=248) | |
|---|---|---|---|
| N (%)a | N (%) | χ2 p-value | |
| Diagnosis (Thal) b | 59 (57.3) | 92 (37.1) | <0.001 |
| Gender (Male) | 61 (62.1) | 101 (40.7) | <0.001 |
| Alcohol Intake (Any in last year) | 62 (62.0) | 90 (36.6) | <0.001 |
| Smoking History (Ever smoked) | 31 (31.0) | 46 (18.7) | 0.013 |
| Sex Hormone Replacement | 24 (23.3) | 24 (9.7) | 0.001 |
| Hypothyroidism | 11 (11.1) | 7 (2.8) | 0.002 |
| Hypogonadism | 28 (27.2) | 36 (14.5) | 0.005 |
| Current Bisphosphonate Use | 11 (10.7) | 11 (4.5) | 0.029 |
| Growth Hormone Use (Past or Present) | 10 (10.1) | 14 (5.7) | NS |
| Initiated Chelation Therapy ≥ 6 years | 62 (80.5) | 136 (70.1) | NS |
| Height Z-score ≤ −2.5 | 23 (22.6) | 43 (17.3) | NS |
| Mean ± SD | Mean ± SD | t-test, p-value | |
| Age, years | 28.1 ± 10.7 | 23.8 ± 11.3 | 0.001 |
| Age of Transfusion Initiation, years | 10.5 ±12.5 | 8.5 ± 10.6 | NS |
| Transfusion Duration, years | 17.2 ± 10.9 | 14.1 ± 9.1 | 0.009 |
| Age of Chelation Initiation, years | 12.5 ±9.7 | 10.9 ±9.3 | NS |
| Chelation Duration, years | 13.7 ±9.4 | 10.7 ±7.8 | 0.007 |
| Serum Ferritin, ng/mL | 4275 ±2792 | 3889 ±2751 | NS |
Percentages included in this table are column percentages.
For categorical variables, the reference variable is provided in parentheses, for example, Thal subjects are the reference compared to TxSCD.
There was a strong, stepwise relationship between alcohol consumption and fracture prevalence. Only 20.1% of subjects who abstain from alcohol suffered fractures compared to 33.5% of those who consume alcohol 1 to 2 times/month, and 51.4% who consume alcohol on a weekly basis (p<0.001). Similarly there was a stepwise increase in fracture prevalence in those subjects who never smoked (25% with fracture), previously smoked (36.4% with fracture) and currently smoked (42.3%, p=0.021)
In multivariate analysis, significant predictors of fracture prevalence were Thal diagnosis (Odds Ratio: 2.3; 1.2–4.5; 95%CI), male gender (OR: 2.6; 1.5–4.4), hypothyroidism (OR: 3.4; 1.2–9.5) and age (OR: 1.1; 1.03–1.08). Transfusion duration was not a significant predictor. There was a weak interaction term between gender and hypogonadism and fracture prevalence. The prevalence of fracture is 2.0 greater among individuals with hypogonadism regardless of gender (p<0.05), whereas for those with normal gonadal function, males have a 2.3 fold greater odds of fracture compared to females (p<0.001).
Discussion
These data suggest that despite similar iron burden, transfused patients with Thalassemia are at greater risk for overall bone fracture as well as atraumatic fracture than transfused subjects with SCD. We previously reported that patients with thalassemia are also at greater risk for endocrinopathy [20], which appears to be related to the increased risk for fracture. In contrast, transfused subjects with SCD had no greater risk of fracture or endocrinopathy compared to non-transfused SCD.
Fracture prevalence in this cohort of iron-overloaded patients with Thal (38.8%) was very similar to that published recently from the Thalassemia Clinical Research Network of North America [6], where 40.6% of patients with Thalassemia major suffered fracture in their lifetime. Similar self-reporting methodology was used in both studies. In this cohort selected for extremely high iron levels, the majority of Thal suffered at least one iron-associated endocrinopathy, which was highly related to the prevalence of fracture. Whereas in the TCRN report [6,7], fracture prevalence was related to low bone mass and sex hormone replacement therapy, but not to the presence of other endocrinopathies.
Subjects with Thalassemia suffered their first fracture at a younger age and were more likely to have multiple fractures compared to TxSCD. Additionally, we found the strongest determinant of new fracture was a previous fracture. Male subjects with transfusion dependent thalassemia tend to fracture more than females, they also are more likely to be hypogonadal [20]. Therefore male patients with thalassemia, endocrine dysfunction and fracture history are at particular risk for future fracture and practitioners should be aware of this risk.
There are very few reports of fracture in SCD subjects with which to compare these data. There is a case report of a 24 year old female with SCD who suffered a stress fracture of the femur from minimal trauma [23], and another of an atraumatic vertebral fracture [24], both rare occurrences. Additionally, in 1986, pathological fracture was reported as a frequent complication in a cohort of SCD patients with osteomyelitis [25]. Despite the paucity of reports regarding fracture, low bone mass is a commonly cited morbidity in this population [10,26,27, 28].
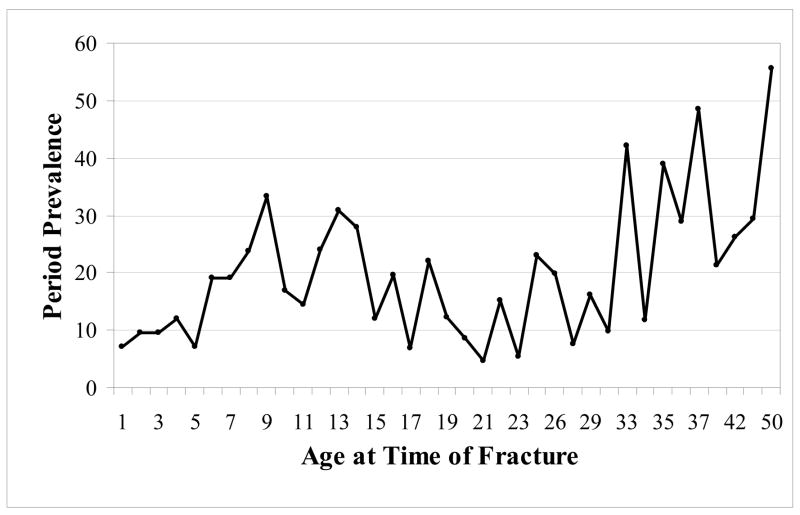
Given this cohort of subjects with hemoglobinopathies is relatively young, median age of 25 years, we would expect few fractures to occur as a result of non-traumatic, non-sports related injuries. Over half of the reported fractures in this study were from a fall, whereas less than a quarter occurred during recreational activities. The most frequently reported fracture site in this study, the upper extremity, was similar to reports in healthy adolescents [3]. Though a much smaller percentage of all fractures (10%), spine, hip and pelvic fractures were relatively high compared to a 1997 report from England in which there were no such reports in patients less than 55 years of age [1]. Moreover the prevalence of fracture in patients less than 18 years of age was lower than published values of fracture prevalence in children without chronic disease [29]. Peak incidence of fracture in a population based cohort from Britain was 14 years for boys and 11 years for girls [3], whereas the peak age of fracture for patients with hemoglobinopathies appears to be in the mid to late 30’s (Figure 2).
Figure 2.
Period prevalence (#fractures/1,000 person-years at risk) for the combined group of subjects with hemoglobinopathies, n=420. There does not appear to be an increase in fracture during the adolescent growth spurt, as is typically observed in healthy reference cohorts.
The percentage of subjects who remain fracture free by the age of 18 years is significantly higher than population estimates of healthy children without hemoglobinopathies [29]. In the cohort of healthy subjects from New Zealand, 50% of children reach 18 years fracture free, while in this combined cohort, 64% of all subjects are fracture free by that age. There does not appear to be an increase in fracture prevalence during the adolescent growth spurt (Figure 2) or surrounding the initiation of menstruation (data not shown), as is typically observed in healthy reference cohorts. Though age at which fracture occurs might be skewed away from adolescence, overall period prevalence by age group appears similar to healthy Caucasian cohorts reported previously [30].
These findings suggest that the epidemiology of fracture is quite different. Fractures occur in young patients with hemoglobinopathies not from risk taking behavior but perhaps secondary to low bone mass or vitamin D deficiency. Though assessment of bone density was uncommon in many of the subjects enrolled in this study, low bone mass has been reported previously in both Thal [6] and SCD [26–28]. BMD Z-score has been shown to decrease with age in both conditions [6, 28]. Vitamin D deficiency, data not ascertained in this cohort, has also been reported in both populations [15,16], which is known to be an independent predictor of fracture.
Despite severe anemia, reduced physical activity and exercise tolerance [19], these adolescents and young adults are not protected from fracture. Their delayed pubertal growth spurt, and perhaps a shift in the development of peak bone mass [6] may shift the period prevalence at which fracture occurs to be much older than in healthy cohorts. Low bone mineral density is a well known risk factor for fracture in adults, but has only recently been in childhood cohorts [31]. Childhood fracture has also been linked to milk avoidance [32] and smoking [33]. Though dairy consumption was not measured in this study, reduced calcium intake has been reported previously [12], and despite a small total number of subject who were current smokers (n=52 or 12.7% of the sample), there was a stepwise increase in fracture risk with smoking habits in these subjects. These observations warrant increased vigilance in monitoring and correcting these behaviors when possible.
This study does have limitations, most notably the self-reporting methodology used to ascertain fracture information. Self-reporting was used to collect information vs. adjudication of each fracture by X-ray. Gathering information in this way may be less accurate and/or over represent the number of fractures, particularly minor fractures [34]. To balance this, we deleted what were presumed to be minor fractures: finger, toe and nose fractures prior to analysis. These ‘minor’ fractures were sustained by less than 5% of the sample and did not differ by group. However, 13% of all remaining fractures were reported as either ‘untreated’ or the patient could not remember how the fracture was treated. One may question the validity of these 24 fractures, but there were no differences among groups in reporting this category. In the case of vertebral fractures, the lack of radiologic evaluation likely resulted in an underestimation of these type of fractures as they often go unreported until a X-ray is taken of the spine for some other indication. Given these limitations, comparing these estimates of fracture with previous literature where fractures were confirmed by X-ray must be done with caution.
Potential ethnic differences in bone density, geometry and subsequent fracture risk must also be considered. For example, Kato et al. reported that the relative risk of fracture in African American women enrolled in the New York Women’s Health Study was 0.45 (95%CI: 0.32–0.63) compared to non-African American women [35]. The majority of patients (44%) with Thal in this cohort considered themselves to be white, whereas 91% of the SCD cohort were non-white. Therefore, ethnic differences in fracture risk cannot be ruled out. However, given the paucity of literature regarding fracture risk in patients with sickle cell disease, and the uniqueness of this cohort in which transfused patients with SCD are observed alongside non-transfused subjects, this information is valuable and may serve as a basis for others to build upon. Finally, we had limited power to detect differences in new fracture incidence amongst the groups over the 4 year prospective data collection period, therefore these data were not included in the analysis for this manuscript. During the 4 year period of data collection, there were 17 new fractures in patients with Thal, 11 new fractures in TxSCD and 2 new fractures in NonTxSCD subjects. In order to collect robust incidence rate ratios in these subject populations, future studies would need to either recruit a larger cohort or follow the subjects for a longer period of time.
Conclusions
These data suggest that despite similar iron burden, transfused patients with thalassemia are at greater risk for bone fracture than subjects with sickle cell disease. Despite recent improvements in transfusion and chelation therapy, male subjects with thalassemia and hypothyroidism are at particular risk for fracture. In contrast, transfused subjects with SCD had no greater risk of fracture or endocrinopathy compared to non-transfused SCD. Though ethnic differences in fracture prevalence must also be considered when comparing these unique groups, it appears that endocrine organs may be protected against iron-related organ injury in SCD, which may also provide protection from high incidence of fracture. If thyroid and gonadal dysfunction are identified and corrected early, risk for fracture may be reduced.
Acknowledgments
Supported in part by the NIH grant R01 DK057778, the Pediatric Clinical Research Center at the Children’s Hospital & Research Center Oakland (M01 RR01271) and the Southern California Thalassemia Center Grant (CDC) 1U01DD000309-01. The authors would like to thank our dedicated research nurse coordinators and clinical research associates at each of the 31 participating centers and of course the subjects and their families who participated in the Multi-Center Study of Iron Overload without whom this work would not be possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johansen A, Evans RJ, Stone MD, Richmond PW, Lo SV, Woodhouse KW. Fracture incidence in England and Wales: a study based on the population of Cardiff. Injury. 1997;28(9–10):655–660. doi: 10.1016/s0020-1383(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 2.Landin LA. Epidemiology of Children’s Fractures. J Pediatric Orthopaedics. 1997;6:79–83. doi: 10.1097/01202412-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C, Dennison EM, Leufkens HGM, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britian: a study using the general practice research database. J Bone Min Res. 2004;19(12):1976–81. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 4.Lips P. Epidemiology and predictors of fractures associated with osteoporosis. Am J Med. 1997;103(2A):3S–11S. doi: 10.1016/s0002-9343(97)90021-8. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 6.Vogiatzi MG, Macklin EA, Fung EB, Vichinsky E, Olivieri N, Kwiatkowski J, Cohen A, Neufeld E, Giardina P. Bone disease in the thalassemia syndromes: a still unresolved problem. J Bone Min Res. 2008 doi: 10.1016/j.bone.2005.10.001. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogiatzi MG, Macklin EA, Fung EB, Vichinsky E, Olivieri N, Kwiatkowski J, Cohen A, Neufeld E, Giardina PJ. Prevalence of fractures among the Thalassemia syndromes in North America. Bone. 2006;38:571–575. doi: 10.1016/j.bone.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RG, Segal JB, Ashar BH, Leung S, Ahmed S, Siddique S, Rice T, Lanzkron S. High prevalence and correlates of low bone mineral density in young adults with sickle cell disease. Am J Hematol. 2006;81(4):236–241. doi: 10.1002/ajh.20541. [DOI] [PubMed] [Google Scholar]
- 9.Sadat-Ali M, Al Elq AH. Sickle cell anaemia: is it cause for secondary osteoporosis? West Afr J Med. 2007;26(2):134–137. [PubMed] [Google Scholar]
- 10.Soliman AT, Bererhi H, Darwish A, Alzalabani MM, Wali Y, Ansari B. Decreased bone mineral density in pubertal children with SCD: correlation with growth parameters, degree of siderosis and secretion of growth factors. J Trop Pediatr. 1998;44:194–198. doi: 10.1093/tropej/44.4.194. [DOI] [PubMed] [Google Scholar]
- 11.De Sanctis V. Growth and puberty and its management in thalassaemia. Hormone Res. 2002;58(Suppl):72–79. doi: 10.1159/000064766. [DOI] [PubMed] [Google Scholar]
- 12.Kawchak DA, Schall JI, Zemel BS, Ohene-Frempong K, Stallings VA. Adequacy of dietary intake declines with age in children with sickle cell disease. J Am Diet Assoc. 2007;107(5):843–8. doi: 10.1016/j.jada.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Zemel BS, Kawchak DA, Fung EB, Ohene-Frempong K, Stallings VA. Effect of zinc supplementation on growth and body composition in children with sickle cell disease. Am J Clin Nutr. 2002;75(2):300–307. doi: 10.1093/ajcn/75.2.300. [DOI] [PubMed] [Google Scholar]
- 14.Bekheirnia MR, Shamshirsaz AA, Kamgar M, Bouzari N, Erfanzadeh G, Pourzahedgilani N, Tabatabaie SM, Shamshirsaz AA, Kimiagar M, Ezzati F, Larijani B. Serum zinc and its relation to bone mineral density in beta-thalassemic adolescents. Biol Trace Elem Res. 2004;97:215–224. doi: 10.1385/bter:97:3:215. [DOI] [PubMed] [Google Scholar]
- 15.Napoli N, Carmina E, Bucchieri S, Sferrazza C, Rini GB, Di Fede G. Low serum levels of 25-hyroxy vitamin D in adults affected by thalassemia major or intermedia. Bone. 2006;38:888–892. doi: 10.1016/j.bone.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Buison AM, Kawchak DA, Schall J, Ohene-Frempong K, Stallings VA, Zemel BS. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145(5):622–627. doi: 10.1016/j.jpeds.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar C, Vichinsky E, Neumayr L. Bone and Joint Disease in Sickle Cell Disease. Hematol/Oncol Clin North Amer. 2005;19:929–941. doi: 10.1016/j.hoc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.De Sanctis V, Stea S, Savarino L, Scialpi V, Traina GC, Chiarelli GM, Sprocati M, Govoni R, Pezzoli D, Gamberini R, Rigolin F. Growth hormone secretion and bone histomorphometric study in thalassemic patients with acquired skeletal dysplasia secondary to desferrioxamine. J Pediatr Endocrinol Metab. 1998;11(Suppl 3):827–33. [PubMed] [Google Scholar]
- 19.Moheeb H, Wali YA, El-Sayed MS. Physical fitness indices and anthropometrics profiles in schoolchildren with sickle cell trait/disease. Am J Hematol. 2007;82(2):91–97. doi: 10.1002/ajh.20755. [DOI] [PubMed] [Google Scholar]
- 20.Fung EB, Harmatz PR, Lee PDK, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP the Multi-Centre Study of Iron Overload Research Group. Increased prevalence of iron-overload associated endocrinopathy in thalassemia versus sickle-cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 21.Fung EB, Harmatz P, Milet M, Ballas SK, De Castro L, Hagar W, Owen W, Olivieri N, Smith-Whitley K, Darbari D, Wang W, Vichinsky E the Multi-Center Study of Iron Overload Research Group. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007;82(4):255–65. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 22.Morris N, Udry J. Validation of a self –administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 23.Bahebeck J, Ngowe NM, Monny Lobe M, Sosso M, Hoffmeyer P. Stress fracture of the femur: a rare complication of sickle cell disease. Rev Chir Orthop Reparatrice Appar Mot. 2002;88(8):816–8. [PubMed] [Google Scholar]
- 24.Jaiyesimi F, Pandey R, Bux D, Sreekrishna D, Zaki Y, Krishnamoorthy N. Sickle cell morbidity profile in Omani children. Ann Trop Paediatr. 2002;22(1):45–52. doi: 10.1179/027249302125000148. [DOI] [PubMed] [Google Scholar]
- 25.Ebong WW. Pathological fracture complicating long bone osteomyelitis in patients with sickle cell disease. J Pediatric Ortho. 1986;6(2):177–81. doi: 10.1097/01241398-198603000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Buison AM, Kawchak DA, Schall JI, Ohene-Frempong K, Stallings VA, Leonard MB, Zemel BS. Bone area and bone mineral content deficits in children with sickle cell disease. Pediatrics. 2005;116:943–949. doi: 10.1542/peds.2004-2582. [DOI] [PubMed] [Google Scholar]
- 27.Sarrai M, Duroseau H, D’Augustine J, Moktan S, Bellevue R. Bone mass density in adults with sickle cell disease. Br J Haematol. 2007;136(4):666–672. doi: 10.1111/j.1365-2141.2006.06487.x. [DOI] [PubMed] [Google Scholar]
- 28.Lal A, Fung EB, Pakbaz Z, Hackney-Stephens E, Vichinsky EP. Bone mineral density in children with sickle cell anemia. Pediatr Blood Cancer. 2006;47:901–906. doi: 10.1002/pbc.20681. [DOI] [PubMed] [Google Scholar]
- 29.Jones IE, Williams SM, Dow N, Goulding A. How many children remain fracture-free during growth? A longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study Osteoporos Int. 2002;13:990–995. doi: 10.1007/s001980200137. [DOI] [PubMed] [Google Scholar]
- 30.Johansen A, Evans RJ, Stone MD, Richmond PW, Lo SV, Woodhouse KW. Fracture incidence in England and Wales: a study based on the population of Cardiff. Injury. 1997;28(9–10):655–660. doi: 10.1016/s0020-1383(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 31.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117(2):e291–7. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr. 2003;77:257–265. doi: 10.1093/ajcn/77.1.257. [DOI] [PubMed] [Google Scholar]
- 33.Jones IE, Williams SM, Goulding A. Associations of birth weight and length, childhood size, and smoking with bone fractures during growth: evidence from a birth cohort study. Am J Epidemiol. 2004;159(4):343–350. doi: 10.1093/aje/kwh052. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 35.Kato I, Toniolo P, Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Riboli E. Diet, smoking and anthropometric indices and postmenopausal bone fractures: a prospective study. Int J Epidemiol. 2000;29(1):85–92. doi: 10.1093/ije/29.1.85. [DOI] [PubMed] [Google Scholar]