Abstract
The wobble base of Escherichia coli elongator tRNAMet is modified to N4-acetylcytidine (ac4C), which is thought to ensure the precise recognition of the AUG codon by preventing misreading of near-cognate AUA codon. By employing genome-wide screen of uncharacterized genes in Escherichia coli (‘ribonucleome analysis'), we found the ypfI gene, which we named tmcA (tRNAMet cytidine acetyltransferase), to be responsible for ac4C formation. TmcA is an enzyme that contains a Walker-type ATPase domain in its N-terminal region and an N-acetyltransferase domain in its C-terminal region. Recombinant TmcA specifically acetylated the wobble base of E. coli elongator tRNAMet by utilizing acetyl-coenzyme A (CoA) and ATP (or GTP). ATP/GTP hydrolysis by TmcA is stimulated in the presence of acetyl-CoA and tRNAMet. A mutation study revealed that E. coli TmcA strictly discriminates elongator tRNAMet from the structurally similar tRNAIle by mainly recognizing the C27–G43 pair in the anticodon stem. Our findings reveal an elaborate mechanism embedded in tRNAMet and tRNAIle for the accurate decoding of AUA/AUG codons on the basis of the recognition of wobble bases by the respective RNA-modifying enzymes.
Keywords: N 4-acetylcytidine (ac4C), RNA acetyltransferase, TmcA, tRNA, wobble modification
Introduction
RNA molecules are decorated by various post-transcriptional modifications. To date, more than 100 species of modified nucleosides have been identified in RNA molecules from all domains of life (Rozenski et al, 1999; Grosjean, 2005; Dunin-Horkawicz et al, 2006). The majority of these RNA modifications were identified and characterized in tRNA molecules. In particular, RNA modifications at the first (wobble) position of the tRNA anticodon participate in the precise decoding of the genetic code that is mediated by the codon–anticodon interaction (Bjork, 1995; Yokoyama and Nishimura, 1995; Curran, 1998; Suzuki, 2005).
N4-acetylcytidine (ac4C) (Figure 1A) is a modified nucleoside that was identified at position 34 (the wobble position) of Escherichia coli elongator tRNAMet in 1972 (Oashi et al, 1972). It is known that ac4C is widely present in a variety of tRNAs and rRNAs; it is present at the wobble position of bacterial tRNAMet and archaeal tRNAs (Gupta, 1984; Sprinzl and Vassilenko, 2005) and is found only at position 12 in eukaryotic tRNAs (Sprinzl and Vassilenko, 2005). In rRNAs, ac4C was found in 5S rRNA from Pyrodictium occultum (Bruenger et al, 1993), in the 3′-terminal helix of 18S rRNAs from Dictyostelium discoideum (McCarroll et al, 1983) and rat liver (Thomas et al, 1978). It is known that ac4C favours the C3′-endo form of its ribose puckering (Kawai et al, 1989), conferring stable codon–anticodon pairing at the wobble position of bacterial tRNAMet. In fact, a biochemical study using in vitro protein synthesis indicated that ac4C prevents misreading of the AUA codon by E. coli tRNAMet (Stern and Schulman, 1978). However, the biogenesis and functions of ac4C in the cell are not fully understood. In Saccharomyces cerevisiae, TAN1 was identified as a protein that is required for ac4C formation at position 12 of tRNASer (CGA) (Johansson and Byström, 2004). Although TAN1 contains the THUMP domain that presumably binds to tRNA, TAN1 seems to lack a catalytic domain for ac4C formation. This indicates the requirement of an unknown partner enzyme for this reaction. Thus, it remains unknown what the acetyl donor is and how the enzyme catalyses the acetylation of RNAs.
Figure 1.
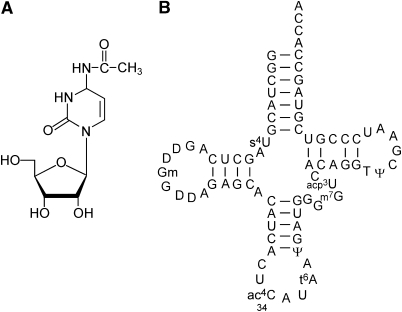
Chemical structure of N4-acetylcytidine (ac4C) and secondary structure of E. coli elongator tRNAMet. (A) Chemical structure of ac4C. (B) Secondary structure of E. coli elongator tRNAMet with modified nucleosides: 4-thiouridine (s4U), 2′-O-methylguanosine (Gm), dihydrouridine (D), N4-acetylcytidine (ac4C), N6-threonylcarbamoyladenosine (t6A), pseudouridine (Ψ), 7-methylguanosine (m7G), 3-(3-amino-3-carboxypropyl) uridine (acp3U) and 5-methyluridine (m5U).
In the bacterial decoding system for AUR (R=A or G) codons, there are two structurally similar tRNAs with the CAU anticodon: tRNAIle for the AUA codon and the elongator tRNAMet for the AUG codon (Figure 1B). Both tRNAs bear specific wobble modifications. The AUA codon-specific tRNAIle contains lysidine (L, k2C) at the first letter of the anticodon, whereas elongator tRNAMet has ac4C at the same position. It is known that L is an essential modification that determines both the codon and amino-acid specificities of tRNAIle (Muramatsu et al, 1988; Soma et al, 2003). Hence, the wobble modification of each tRNA strictly governs its identity and decoding accuracy. Each of these tRNAs has identity elements embedded in its sequence and tertiary structure that are recognized by an RNA-modifying enzyme for the wobble position and by a cognate aminoacyl-tRNA synthetase (Ikeuchi et al, 2005). For lysidine formation in tRNAIle, we previously identified the tRNAIle-lysidine synthetase (tilS) that strictly discriminates tRNAIle from tRNAMet by recognizing two consecutive base pairs in the acceptor stem (Soma et al, 2003; Ikeuchi et al, 2005). If ac4C is accidentally introduced at the wobble position of tRNAIle, tRNAIle loses its isoleucylation and AUA-decoding abilities. Thus, it was speculated that tRNAIle also has another set of determinants negatively recognized by a putative enzyme responsible for the ac4C formation that occurs in elongator tRNAMet.
To identify genes responsible for RNA modifications from uncharacterized genes, we have frequently employed a reverse genetic approach combined with mass spectrometry (‘ribonucleome' analysis) (Suzuki, 2005; Ikeuchi et al, 2006; Noma and Suzuki, 2006; Noma et al, 2006; Suzuki et al, 2007). This analysis utilizes a series of gene-deletion strains of E. coli or S. cerevisiae. The total RNA extracted from each strain is analysed by liquid chromatography/mass spectrometry (LC/MS) to determine whether a particular gene deletion leads to the absence of a specific modified base, thereby permitting us to identify the enzyme or protein responsible for this modification. In the case of essential genes, we analyse either temperature-sensitive mutants cultured at the non-permissive temperature or expression-controlled strains. This ribonucleome analysis enables us to identify not only enzyme genes directly responsible for RNA modifications, but also genes that encode non-enzymatic proteins necessary for the biosynthesis of RNA modifications. These include carriers of the metabolic substrates used for RNA modifications and partner proteins needed for RNA recognition. In fact, using this approach, we previously identified tilS, an essential gene for lysidine formation (Soma et al, 2003), tusA-E for 2-thiouridine formation (Ikeuchi et al, 2006) and TYW1-4 for wybutosine synthesis (Noma et al, 2006).
Here, we used ribonucleome analysis to identify and characterize a gene, which we named tmcA (tRNAMet cytidine acetyltransferase), responsible for ac4C formation in the E. coli elongator tRNAMet. Biochemical analyses revealed mechanistic insights into ac4C formation and how TmcA discriminates elongator tRNAMet from the structurally similar tRNAIle.
Results
Ribonucleome analysis identified the ypfI gene to be required for ac4C formation
To identify a gene responsible for ac4C formation in E. coli, we employed a genome-wide screen of a series of knockout strains using the ribonucleome analysis (Suzuki, 2005; Ikeuchi et al, 2006; Suzuki et al, 2007). If the strain contains a deleted gene encoding an enzyme or protein involved in RNA modification, the absence of a specific modified nucleoside can be identified by LC/MS analysis. Initially, we analysed 130 genomic-deletion strains covering ∼50% of E. coli genes, each of which lacked about 20 kbps (∼20 genes) (Baba et al, 2006). In the analysis, we found a strain OCL58 that specifically lacks ac4C (data not shown). The deleted genomic region of OCL58 spans ypfN to yfgD (55.84–56.38 min) and contains 24 genes.
To narrow down the target gene, we employed a computational domain search by Pfam (Finn et al, 2006) to characterize these candidates. As a result, the ypfI gene was found to have an N-acetyltransferase domain (Acetyltransf_1) belonging to the GCN5-related histone acetyltransferase family (GNAT family). We constructed a single knockout strain of ypfI (ΔypfI). Nucleosides analysis by LC/MS of the total RNA obtained from the ΔypfI strain revealed the specific absence of ac4C (Figure 2A), demonstrating that ypfI is an essential gene for ac4C formation in the cell. Despite the absence of ac4C, the ΔypfI strain showed a healthy phenotype without any growth defects compared with wild-type cells (data not shown). When the ΔypfI strain was cocultivated with wild-type cells, no difference in the survival rate could be observed (data not shown).
Figure 2.
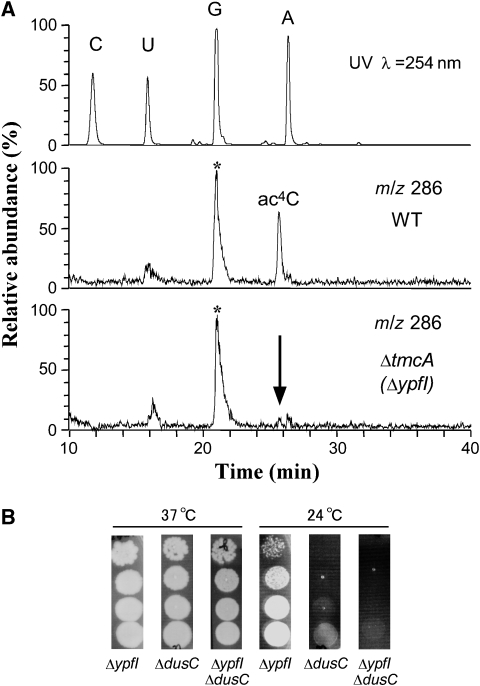
Mass spectrometric analysis of total nucleosides from the ΔypfI strain and growth phenotype of the ΔypfI/ΔdusC strain. (A) LC/MS analysis of total nucleosides in the wild-type (WT) and ΔypfI strains. The top panel is the UV trace at 254 nm of the WT strain. The middle (WT) and bottom (ΔypfI) panels are mass chromatograms at m/z 286 for detecting a proton adduct (MH+) of ac4C. Absence of ac4C in ΔypfI strain is indicated by the arrow. Asterisks indicate isotopic ions of guanosine. (B) Growth property of the ΔypfI/ΔdusC strain. Each deletion strain was serially diluted (1:10 dilutions) and then spotted onto LB plates and incubated at 37 and 24 °C for 2 days.
Non-essential modifications are known to have an important function in tRNA stability in the cell (Alexandrov et al, 2006; Chernyakov et al, 2008). We next sought to observe the synthetic phenotype of ΔypfI when it was combined with additional deletions of genes responsible for biogenesis of other modified nucleosides in tRNAMet. We chose nine deletion strains, ΔthiI, ΔdusA, ΔdusB, ΔdusC, ΔtrmH, ΔtruA, ΔtrmB, ΔtrmA and ΔtruB, each of whose deletion was transferred to ΔypfI by P1 transduction, to construct a series of double-deletion strains. No significant growth phenotype was seen in any of the double-deletion strains when cultured at 37 °C. However, when cultured at 24 °C, the ΔypfI/ΔdusC strain showed a severe growth defect compared with each of the single-deletion strains (Figure 2B). The gene dusC encodes an enzyme responsible for dihydrouridine formation in the D-loop of tRNAs (Bishop et al, 2002). The ΔdusC mutant originally showed a cold-sensitive phenotype, and the additional deletion of ypfI enhanced this phenotype.
Reconstitution of ac4C formation in vitro using recombinant TmcA
We found apparent homologues of E. coli YpfI in γ-proteobacteria, including Salmonella typhimurium, Yersinia pestis, Haemophilus influenzae, Pasteurella multocida and Vibrio cholerae (Figure 3). Sequence alignment of YpfI showed that these proteins shared many conserved regions. The N-terminal region contains the uncharacterized DUF699 domain (PF05127). DUF699 purportedly functions as a putative ATPase, bearing the highly conserved ATP/GTP-binding motif (P-loop) known as the Walker A motif (AxRGRGKT/S) and the Walker B motif (hhhhDEAA) (Figure 3). The C-terminal Acetyltransf_1 domain (PF00583) is a member of the GNAT family.
Figure 3.
Sequence alignment of TmcA from γ-proteobacteria. Amino-acid sequence alignment of YpfI in E. coli (ECO) and homologues in other bacteria (STY, S. typhimurium LT2; YPE, Y. pestis; HIN, H. influenzae R3021; PMU, P. multocida; and VCH, V. cholerae) were aligned. Two domains (DUF699 and Acetyltransf_1) predicted by Pfam are indicated by black solid and dotted lines, respectively. The Walker A motif is indicated by grey line. The black and grey boxes indicate a degree of sequence similarity.
The structural characteristics of YpfI prompted us to speculate that acetyl-CoA and ATP are required for ac4C formation. To characterize the E. coli YpfI protein and to reconstitute ac4C formation in vitro, a hexa-histidine-tagged E. coli YpfI was expressed and purified. We then tried to reconstitute ac4C formation at the wobble position of in vitro-transcribed tRNAMet in the presence or absence of acetyl-CoA and ATP. After the reaction, total nucleosides of the tRNA substrate for each reaction were analysed by LC/MS. As shown in Figure 4A and B, ac4C clearly appeared only when the reaction was performed in the presence of both acetyl-CoA and ATP. No ac4C formation occurred under conditions without acetyl-CoA or ATP. This data demonstrates that YpfI is an acetyltransferase responsible for ac4C formation at the wobble position of tRNAMet. We therefore renamed ypfI as tmcA (tRNAMet cytidine acetyltransferase).
Figure 4.
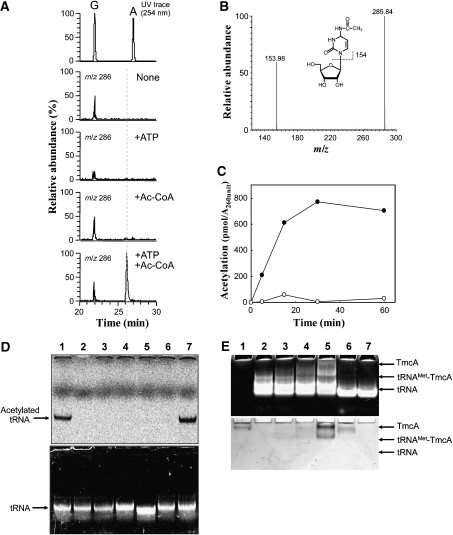
Reconstitution of ac4C formation in vitro using recombinant TmcA. (A) Mass spectrometric detection of ac4C formation in the unmodified tRNAMet that was transcribed in vitro. After the reaction, the tRNAMet was digested into nucleosides and analysed by LC/MS. Mass chromatograms at m/z 286 detected ac4C, which was reconstituted in vitro in the absence of both ATP and acetyl-CoA (second panel), in the presence of ATP (third panel), in the presence of acetyl-CoA (fourth panel) or in the presence of both ATP and acetyl-CoA (bottom panel). The ac4C appeared only when both substrates were present. (B) Mass spectrum of ac4C that was reconstituted in vitro. (C) In vitro reconstitution of ac4C formation in the presence (filled circle) or absence (open circle) of ATP, which was detected by liquid scintillation counting of 14C-acetyl group. (D) In vitro reconstitution of ac4C formation under various conditions, the radioactivity of which was visualized by an imaging analyser (FLA-7000, Fujifilm). The upper and lower panels show visualized radioactivity and ethidium bromide staining. The reaction was performed in the presence of ATP (lane 1), GTP (lane 7) and ADP (lane 6), in the absence of ATP (lane 3) and TmcA (lane 2). The mutant tRNAMet, EM(C34G), whose C34 was replaced by G34 (lane 4) and tRNAIle2 (lane 5), were employed for ac4C reconstitution instead of tRNAMet. (E) Gel-retardation analysis of tRNAMet with recombinant TmcA. The polyacrylamide gel was stained by ethidium bromide (upper panel) and Coomassie brilliant blue (lower panel). Lane 1, TmcA (80 pmol); lane 2, tRNAMet; lane 3, TmcA (40 pmol) and tRNAMet; lane 4, TmcA (80 pmol) and tRNAMet; lane 5, TmcA (160 pmol) and tRNAMet; lane 6, TmcA (80 pmol) and tRNAIle2; lane 7, tRNAIle2. Amount of tRNA was 80 pmol in all conditions.
Reconstitution of ac4C formation in vitro was further analysed by a filter assay. The substrate tRNAMet was acetylated by the recombinant TmcA in the presence of [1-14C] acetyl-CoA and ATP. As shown in Figure 4C, ATP-dependent acetylation could be confirmed. To quantify the acetylated tRNA on the filter by liquid scintillation counting, we first had to remove the free [1-14C] acetyl-CoA by phenol extraction and ethanol precipitation, due to its high background signal (see Experimental procedures). Therefore, we did not attempt to measure initial velocity of the acetylation, which is necessary to determine the exact kinetic parameters of ac4C formation. The radioactivity of 14C-labelled acetylated tRNAs was visualized on a gel (Figure 4D). In this experiment, TmcA did not acetylate tRNAIle2 as well as tRNAMet with a C34G mutation. According to the gel-mobility shift experiment (Figure 4E), TmcA specifically interacts with tRNAMet. These data suggest that TmcA strictly recognizes the wobble base and discriminates tRNAMet from the structurally similar tRNAIle2.
We also found that TmcA can utilize GTP in place of ATP for ac4C formation (Figure 4D). It is known that some enzymes bearing a P-loop motif (Walker A motif) utilize GTP as a substrate instead of ATP (Saraste et al, 1990). Therefore, we considered whether GTP is also a natural substrate for TmcA. We employed ADPCP and GDPCP, which are non-hydrolyzable analogues of ATP and GTP, for ac4C formation, and found that these analogues could not produce ac4C (data not shown). This demonstrated that TmcA requires the hydrolysis of ATP or GTP for ac4C formation.
Kinetic analysis of ATP and GTP hydrolysis by TmcA
To gain mechanistic insights into ac4C formation driven by ATP/GTP hydrolysis, the ATPase (or GTPase) activity of TmcA was characterized. We employed [α-32P]-labelled ATP (or [α-32P]-labelled GTP) as a substrate to examine and quantify the products released by the ATP/GTP hydrolysis catalysed by TmcA. TmcA hydrolysed ATP or GTP between the β- and γ-phosphates, as shown by the detection of labelled ADP (or GDP) on the polyethtyleneimine (PEI)-cellulose thin-layer chromatography (Supplementary Figure S1). The kinetic parameters of ATP (or GTP) hydrolysis were measured in the presence or absence of tRNAMet and acetyl-CoA (Table I). Even in the absence of both acetyl-CoA and tRNAMet, TmcA intrinsically hydrolysed ATP and GTP, indicating that ATP/GTP hydrolysis by TmcA is an independent reaction from that of ac4C formation by the acetyltransferase.
Table 1.
Kinetic parameters for ATP/GTP hydrolysis
| NTP | tRNAMet (2.5 μM) | Acetyl-CoA (200 μM) | Km (μM) | kcat (10−3 s−1) | kcat/Km (10−3 s−1 μM−1) |
|---|---|---|---|---|---|
| ATP | − | − | 2.93 | 8.07 | 2.75 |
| − | + | 3.20 | 22.2 | 6.95 | |
| + | − | 2.79 | 16.4 | 5.88 | |
| + | + | 4.16 | 29.5 | 7.10 | |
| GTP | − | − | 117 | 25.2 | 0.215 |
| − | + | 88.7 | 63.3 | 0.714 | |
| + | − | 107 | 28.7 | 0.267 | |
| + | + | 66.6 | 53.7 | 0.807 |
The Km value of ATP hydrolysis (2.93 μM) was markedly lower than that of GTP hydrolysis (117 μM) in the absence of both acetyl-CoA and tRNAMet. Addition of acetyl-CoA (200 μM) to the reaction stimulated the kcat value of ATP (2.8-fold) and GTP (2.5-fold) hydrolyses. In the presence of tRNAMet (2.5 μM), the kcat value of ATP hydrolysis was doubled, whereas no significant change in the kcat value of GTP hydrolysis was observed. In ATP hydrolysis, the addition of both acetyl-CoA and tRNAMet increased the kcat value 3.7-fold and slightly affected the Km value, resulting in a 2.6-fold increase in kcat/Km. In GTP hydrolysis, the addition of both substrates decreased the Km value 0.57-fold and increased the kcat value 2.1-fold, which resulted in a 3.8-fold increase in kcat/Km. The data reveal that ATP/GTP hydrolysis by TmcA is stimulated by the addition of acetyl-CoA and tRNAMet.
TmcA recognizes the anticodon stem of tRNAMet
In eubacteria, it is difficult to distinguish primary transcripts of elongator tRNAMet and tRNAIle2 because of their high sequence similarity, and especially because they have identical anticodon loop sequences. To explore elements embedded in tRNAMet that are recognized by TmcA, we next employed mutation studies using in vitro-transcribed tRNAs. Various mutants of E. coli elongator tRNAMet and tRNAIle2 were constructed by in vitro transcription (Figure 5A). The relative activity of ac4C formation, which was visualized on a gel by the imaging analyser, was quantified by measuring the radioactivity of the 14C-labelled acetyl group in each variant at the end point of the reaction.
Figure 5.
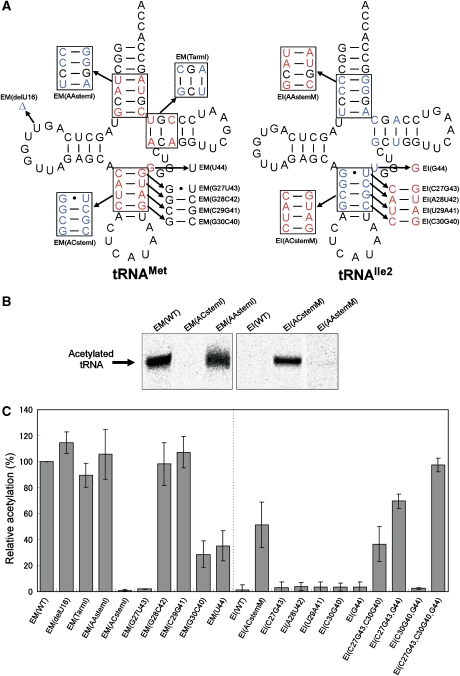
Mutation study of tRNAMet and tRNAIle2 to investigate the positive and negative determinants of ac4C formation. (A) tRNA variants based on E. coli elongator tRNAMet (left-hand side) and E. coli tRNAIle2 (right-hand side) that were used in this study. The numbering system of the tRNA is based on the tRNA compilation by Sprinzl and Vassilenko (2005). Arrows and boxes indicate the substitutions and deletions made in this study. EM and EI stand for E. coli elongator tRNAMet and E. coli tRNAIle2, respectively. (B) Detection of ac4C formation in tRNA variants visualized by the imaging analyser. (C) Relative acetylation activities of a series of tRNA variants. Radioactivity of 14C-acetylation on each tRNA was quantified by FLA-7000 system (Fujifilm). Radioactivity of ac4C in EM(WT) was standardized as 100%. A full-colour version of this figure is available at The Embo Journal Online.
As most of the differences between elongator tRNAMet and tRNAIle2 can be seen in their acceptor and anticodon stems, we first swapped each of these stems in the two tRNAs. When their acceptor stems were exchanged, no change in the specificity was observed in either mutant (EM(AAstemI) and EI(AAstemM)) (Figure 5B). However, when their anticodon stems were exchanged, no acetylation was detected in mutant tRNAMet bearing the anticodon stem of tRNAIle2 (EM(ACstemI)) (Figure 5B). On the other hand, the mutant tRNAIle2 bearing the anticodon stem of tRNAMet (EI(ACstemM)) acquired acetylation capability (Figure 5B). The only difference in the D arms between tRNAMet and tRNAIle2 is the absence of a uridine at position 16 of tRNAIle2. However, U16 deletion of tRNAMet (EM(delU16)) did not affect ac4C formation (Figure 5C). Next, we replaced the T arm of tRNAMet with that of tRNAIle2 (EM(TarmI)), but no significant change in ac4C formation was observed (Figure 5C). These results reveal that TmcA specifically recognizes the anticodon stem of tRNAMet.
To identify which bases in the anticodon stem are important for ac4C formation, base pairs in the anticodon stems of these tRNAs were interexchanged. When the top pair (C27-G43) of tRNAMet was replaced with G27·U43 of tRNAIle2, the mutant tRNAMet (EM(G27U43)) was not acetylated (Figure 5C), suggesting that the C27-G43 pair of tRNAMet is critical for ac4C formation. However, as mutant tRNAIle2 bearing C27-G43 (EI(C27G43)) was not acetylated (Figure 5C), C27-G43 is not sufficient for ac4C formation on tRNAIle2. Swapping the second base pair (EM(G28C42) and EI(A28U42)) and the third base pair (EM(C29G41) and EI(U29A41)) in the anticodon stem did not influence acetylation (Figure 5C). When C30-G40 of tRNAMet was replaced by G30-C40 of tRNAIle2, we saw a considerable reduction in ac4C formation (EM(G30C40)) (Figure 5C). In addition, when G44 of tRNAMet was replaced by U44 of tRNAIle2, the mutant tRNAMet (EM(U44)) showed a slight reduction in acetylation. However, neither C30-G40 nor G44 of tRNAMet acted as positive determinants for ac4C formation on tRNAIle2 (EI(C30-G40) and EI(G44)) (Figure 5C).
We next constructed tRNAIle2 mutants bearing the two positive elements for ac4C formation, to determine the minimum necessary elements for the conversion of the specificity of the wobble modification from lysidine to ac4C. When C27-G43 and C30-G40 were introduced into tRNAIle2 simultaneously, the mutant tRNAIle2 (EI(C27G43, C30G40)) was acetylated (Figure 5C). In addition, introduction of C27-G43 and G44 into tRNAIle2 (EI(C27G43, G44)) also conferred a specificity for TmcA (Figure 5C). However, when C30-G40 and G44 were simultaneously introduced into tRNAIle2 (EI(C30G40, G44)), no ac4C formation was observed (Figure 5C). Introduction of C27-G43 is a critical, but insufficient, element for ac4C formation on tRNAIle2. Additional introduction of C30-G40 or G44 with the C27-G43 mutation is therefore required for the acetylation of tRNAIle2. Finally, when these three elements were introduced simultaneously (EI(C27G43, C30G40, G44)), full acetylation was observed (Figure 5C).
Discussion
Here, we successfully identified the RNA acetyltransferase (TmcA) responsible for ac4C formation at the wobble position of tRNAMet. Despite the purported function of ac4C in preventing misreading of the AUA codon, at least in in vitro translation (Stern and Schulman, 1978), a healthy phenotype for the ΔtmcA strain was unexpectedly observed. Thus, ac4C is a dispensable modification of tRNAMet, at least in the presence of the AUA codon-specific tRNAIle2. The only phenotype we observed in this study was a cold-sensitive phenotype for the double-deletion strain (ΔtmcA/ΔdusC) (Figure 2B). We have no plausible explanation why the lack of ac4C in tRNAMet caused a growth defect at low temperatures in the absence of dihydrouridine. In eukaryotic tRNAs, it is known that non-essential modifications are required for tRNA stability in the cell (Alexandrov et al, 2006). In addition, recent studies have revealed that RNA metabolism is involved in the rapid degradation of hypomodified tRNAs (Chernyakov et al, 2008; Wang et al, 2008). Further study is necessary to reveal whether a mechanism similar to eukaryotic tRNA metabolism is involved in the E. coli system. It is also possible to speculate that a functional defect of the hypomodified tRNAMet in the double-deletion strain causes a functional defect in protein synthesis at low temperature. On the other hand, in some pathogenic microorganisms, it is known that RNA modifications have an important function in virulence expression (Durand et al, 1994, 1997; Takano et al, 2006). From the viewpoint of virulence in γ-proteobacteria, the cellular function of ac4C remains to be investigated.
TmcA belongs to COG1444 in the Clusters of Orthologous Groups gene database (Tatusov et al, 2001). Although homologues of TmcA occur in many Archaea and Eukarya, in bacteria the gene appears to be limited to the γ-proteobacterial subphylum. Consistently, ac4C was not reported at the wobble position in other sequenced tRNAsMet from Bacillus subtilis, Mycoplasma capricolum or Thermus thermophilus, which are not γ-proteobacteria (Sprinzl and Vassilenko, 2005). The limited distribution of TmcA and ac4C at the wobble position of tRNAMet in γ-proteobacteria could be caused by the loss of tmcA in other bacteria. Otherwise, tmcA might have arose in a common ancestor of γ-proteobacteria by horizontal gene transfer from other domains of life. Consistent with this, tmcA homologues are widely distributed in archea and eukaryotic species (KOG2036 in eukaryotic cog database). It is known that ac4C occurs at the wobble positions of several tRNAs in archea (Gupta, 1984), and at position 12 in a subset of tRNAs in eukaryotes (Sprinzl and Vassilenko, 2005). In addition, it has been reported that 18S rRNA contains ac4C in its 3′-terminal region in some eukaryotes (Thomas et al, 1978; McCarroll et al, 1983). Further studies should reveal whether eukaryotic homologues of tmcA are actually involved in ac4C formation on tRNA or rRNA.
TmcA contains an ATPase domain in its N-terminal region and an N-acetyltransfease domain related to the histone acetyltransferase family in its C-terminal region. In vitro reconstitution of ac4C formation revealed that TmcA specifically acetylates C34 of elongator tRNAMet by using acetyl-CoA as an acetyl donor, and this reaction requires ATP/GTP hydrolysis. Unlike histone acetyltransferases, TmcA was found to be an energy-consuming acetyltransfease. ATP hydrolysis had a higher kcat/Km value (7.10 × 10−3 s−1 μM−1) than that of GTP hydrolysis (0.807 × 10−3 s−1 μM−1) (Table I). Considering the cellular concentration of ATP (3 mM) and GTP (0.9 mM) in E. coli (Bochner and Ames, 1982) and the Km values of ATP/GTP hydrolysis by TmcA, ATP would be a more favourable substrate for TmcA than GTP under physiological conditions. According to our kinetic study of ATP/GTP hydrolysis, we observed that TmcA hydrolyses ATP/GTP in the absence of acetyl-CoA and tRNAMet, indicating that TmcA can hydrolyse ATP/GTP independently of its RNA acetylation activity. In the presence of acetyl-CoA or tRNAMet, the kcat value of ATP hydrolysis increased, suggesting a functional interplay between ATPase and GNAT domains in TmcA. The mechanisms of ATP hydrolysis-driven ac4C formation needs to be elucidated, although it can be speculated that some conformational change of TmcA induced by the binding of acetyl-CoA or tRNAMet might stimulate its ATPase activity.
There are other cases where wobble modifications require ATP hydrolysis for their biogenesis. TilS (Soma et al, 2003; Ikeuchi et al, 2005) and MnmA (Ikeuchi et al, 2006; Numata et al, 2006) are RNA-modifying enzymes responsible for the formation of lysidine (L) and 2-thiouridine (s2U), respectively. Both enzymes share a common N-type ATP pyrophosphatase catalytic domain (Rizzi et al, 1996; Tesmer et al, 1996; Lemke and Howell, 2001) that contains a different type of P-loop motif (SGG × DS). TilS and MnmA recognize tRNAs and activate the C2 position of the pyrimidine base at position 34 by forming an adenylated intermediate. For lysidine formation by TilS (Ikeuchi et al, 2005), the ɛ-amino group of lysine attacks the activated C2 position of C34 to synthesize lysidine by releasing AMP. Similarly, the persulfide sulphur of MnmA replaces AMP to form 2-thiouridine (Ikeuchi et al, 2006; Numata et al, 2006). In both cases, the α-β phosphate bond of ATP is hydrolysed. As TmcA hydrolyses the β–γ phosphate bond of ATP (or GTP), this indicates that the mechanism of ac4C formation utilizing ATP hydrolysis is completely different from the biogenesis of L or s2U.
The Walker-type ATPase domain is widely found in AAA+ superfamily proteins, which are molecular chaperones for nucleic acids and proteins (Ogura and Wilkinson, 2001), including various motor proteins, the ABC transporter, ATP-dependent proteases and RNA helicases (White and Lauring, 2007). The ATPase module in TmcA might be required to accommodate C34 in the catalytic centre of the GNAT domain by twisting tRNAMet, and/or for the efficient turnover of the reaction by promoting product release.
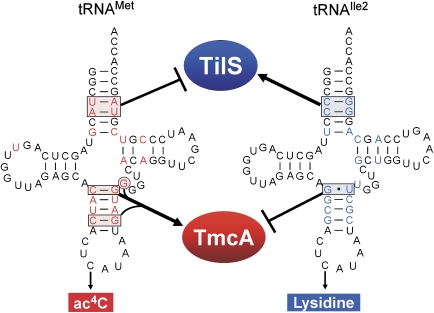
In vitro acetylation of tRNA variants revealed that TmcA discriminates tRNAMet from the structurally similar tRNAIle2 by recognizing C27-G43, C30-G40 and G44 in the anticodon stem. In fact, when these three elements were introduced into tRNAIle2 simultaneously, the mutant tRNAIle2 acquired full acetylation capability (Figure 5C). C27–G43 in tRNAMet is the most critical positive determinant for ac4C formation, and as a mutant tRNAMet having a G27·U43 mutation (EM(G27U43)) completely failed to be acetylated (Figure 5C), G27·U43 of tRNAIle2 is a negative determinant for ac4C formation. We previously reported that TilS positively recognizes two consecutive base pairs (C4-G69 and C5-G68) in the acceptor stem of tRNAIle2 for lysidine formation (Ikeuchi et al, 2005). In contrast, the corresponding base pairs (U4-A69 and A5-U68) in tRNAMet exert an effect as negative determinants of lysidine formation (Ikeuchi et al, 2005). As shown in Figure 6, two sets of determinants for wobble modifications embedded in both tRNAMet and tRNAIle2 create mutual and exclusive recognition sites for two RNA-modifying enzymes. Thus, TmcA and TilS successfully utilize limited differences in these similar tRNAs. In particular, TmcA mainly discriminates differences in bases in the anticodon stem, whereas TilS recognizes base pairs in the acceptor stem. There are no overlaps in the determinants of these two enzymes.
Figure 6.
Recognition and discrimination, by TmcA and TilS, of two tRNAs with CAU anticodons. Schematic depiction of tRNA recognition by TmcA in comparison with TilS. In this picture, the anticodon regions of both tRNAs are not highlighted as positive elements for both enzymes, as this region is commonly recognized by both enzymes. TmcA strongly recognizes C27-G43 in the anticodon stem of tRNAMet. In addition, C30-G40 and G44 in tRNAMet have an important function for TmcA recognition. G27·U43 in tRNAIle2 exerts an effect as a negative determinant for TmcA recognition. On the other hand, TilS strongly recognizes two consecutive base pairs, C4-G69 and C5-G68, in the acceptor stem of tRNAIle2 for lysidine formation, whereas U4-A69 and A5-U68 in tRNAMet exert an effect as negative determinants for TilS recognition.
In summary, this study has elucidated an exquisite mechanism in tRNAMet and tRNAIle for the accurate decoding of AUA/AUG codons on the basis of the recognition of cognate wobble modifications by two RNA-modifying enzymes.
Materials and methods
Strains and media
Series of E. coli genomic-deletion strains (OCL/R-series) derived from MG1655sp (MG1655 rpsL polA12 Zih∷Tn10), each of which lacks about 20 kbp (∼20 genes), were kindly provided by Dr Jun-ichi Kato (Hashimoto et al, 2005). OCL58 (MG1655sp Δ(ypfH- yfgD)∷kan) specifically lacked ac4C. The E. coli K-12 strain BW25113 (lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78) was used for the ‘one-step inactivation of chromosomal genes' procedure (Datsenko and Wanner, 2000; Baba et al, 2006). To amplify DNA fragments of the chloramphenicol acetyltransferase gene (Cmr) with 40-nt extensions at both ends homologous to the flanking sequences of the ypfI gene, pairs of oligo DNAs, ΔypfI-F(Cmr) (5′-gcaatacttttggtaaaagcatttaacttccggggcagggactaaatcagtaagttggcagc-3′) and ΔypfI-R(Cmr) (5′-aagtgcgaacagcgcctgcgcggcctcttcccgctgacggaaccagcaatagacataagcggc-3′) were used to construct BW25113 ΔypfI∷Cm. The pBT vector (Stratagene) was used as a template of Cmr.
A series of single-deletion strains having a kanamycine-resistant marker (Kmr) (ΔypfI, ΔthiI, ΔdusA, ΔdusB, ΔdusC, ΔtrmH, ΔtruA, ΔtrmB, ΔtrmA and ΔtruB) (Baba et al, 2006) were obtained from the Genetic Stock Research Center, National Institute of Genetics, Japan. To construct a series of double-deletion strains (for example, ΔypfI/ΔdusC), ΔypfI∷Cm was transferred to a series of single-deletion strains (Kmr) by P1-transduction. The E. coli strains were grown in 5 ml of LB medium in 24-well plates at 37 °C overnight.
Mass spectrometry
The total RNA was extracted from grown cells using an acid-guanidinium thiocyanate-phenol–chloroform reagent (ISOGEN, Nippon Gene, Japan). The extracted RNAs were digested into nucleosides and analysed by LC/MS using ion-trap mass spectrometry as described previously (Ikeuchi et al, 2006; Noma et al, 2006; Suzuki et al, 2007).
Construction and purification of recombinant protein
Oligonucleotides YpfI-NdeI-F (5′-gagatatacatatggctgaactgactgcgcttcaca-3′) and YpfI-XhoI-R (5′-ggtgctcgaggtgaaataattgccattgcgttatg-3′) were used to amplify ypfI from the E. coli genome by PCR. The PCR products were cloned into the NdeI and XhoI sites of pET21b (Novagen) to generate pET21-TmcA. E. coli BL21(DE3) was used as the host for the expression of recombinant TmcA. The C-terminal 6 × His-tagged TmcA protein was expressed in soluble form by induction with 0.1 mM IPTG. Recombinant TmcA was purified by Ni-charged HiTrap Chelating HP chromatography (GE Healthcare) according to the manufacturer's instructions. The protein concentration was determined with the Bio-Rad protein assay kit using bovine serum albumin as a standard. The pooled protein was dialysed against a buffer consisting of 50 mM Tris–HCl (pH 7.5), 1 mM dithiothreitol and 10 mM KCl. Glycerol was added to the protein solution to a final concentration of 30%, and the solution was frozen quickly in liquid nitrogen and stored at −70°C.
Preparation of tRNA variants
A series of tRNA variants were transcribed in vitro using T7 RNA polymerase as described (Ikeuchi et al, 2005). Templates for in vitro transcription were constructed by PCR using synthetic DNA oligonucleotides carrying the tRNA gene under the T7 promoter sequence (Sampson and Uhlenbeck, 1998). The oligo-DNAs used for the construction of 22 tRNA variants are shown in the Supplementary Table S1. The transcribed tRNAs were gel-purified by running them on 10% polyacrylamide gel containing 7 M urea.
In vitro ac4C formation
ac4C formation was performed at 37 °C in a reaction mixture (50 μl) consisting of 100 mM Tris–HCl (pH 7.8), 10 mM KCl, 10 mM MgCl2, 10 mM DTT, 1 mM ATP, 120 μM [1-14C] acetyl-CoA (American Radiolabeled Chemicals, 55 mCi mmol−1), 5.0 μg of recombinant TmcA protein and 0.04 A260 units (60 pmol) of in vitro-transcribed tRNAMet. An aliquot (10 μl) of reaction mixture taken at various time points was phenol–chloroform-extracted and ethanol-precipitated to remove free [1-14C] acetyl-CoA. The recovered tRNA was spotted onto Whatman 3 MM filter discs. The discs were washed three times with 5% trichloroacetic acid and the radioactivity was measured by liquid scintillation counting.
To visualize the radioactivity of ac4C, the reaction mixture was directly analysed by running it on a 10% polyacrylamide gel containing 7 M urea with 1 × TBE. The gel was stained by ethidium bromide for digital photo recording, dried and the radioactivity of the acetylated tRNAs was visualized and quantified by an imaging analyser (FLA-7000 system, Fujifilm, Japan).
For LC/MS analysis, ac4C formation was performed at 37 °C in a reaction mixture (10 μl) consisting of 100 mM Tris–HCl (pH 7.8), 10 mM KCl, 10 mM MgCl2, 10 mM DTT, 1 mM ATP, 1 mM acetyl-CoA, 1.4 μg of recombinant TmcA and 0.1 A260 units (150 pmol) of in vitro-transcribed tRNAMet. After the reaction, the tRNA was digested into nucleosides with nuclease P1 and bacterial alkaline phosphatase as described (Suzuki et al, 2007) and was directly analysed by LC/MS (Ikeuchi et al, 2006; Noma et al, 2006; Suzuki et al, 2007).
Gel retardation experiment
The gel retardation experiment was basically performed as described (Soma et al, 2003). Recombinant TmcA (40–160 pmol) and in vitro-transcribed tRNA (80 pmol) were incubated at 37 °C for 15 min in a 10 μl buffer consisting of 50 mM Tris–HCl (pH 8.5), 15 mM MgCl2, 5 mM DTT and 1 mM spermine. The complex was run on a 6% native polyacrylamide gel with 50 mM Tris, 5 mM Mg(OAc)2 and 5 mM DTT (pH 7.1, adjusted with acetic acid). After electrophoresis, the gel was stained with ethidium bromide to visualize the tRNA and then stained with Coomasie brilliant blue to visualize the protein.
Kinetic analysis of ATP/GTP hydrolysis
The experiments of ATP/GTP hydrolysis were basically performed as described (Terasaki et al, 2004; Ikeuchi et al, 2005) in a reaction mixture (50 μl) consisting of 100 mM Tris–HCl (pH 7.8), 10 mM KCl, 10 mM MgCl2, 10 mM DTT, 0.1–250 μM ATP with 0.25 μl of [α-32P]ATP (∼3.7 GBq ml−1, GE Healthcare) or 0.1–250 μM GTP with 0.25 μl of [γ-32P]GTP (∼3.7 GBq ml−1, GE Healthcare), and TmcA (50 pmol). To determine the kinetic parameters of ATP/GTP hydrolysis in the presence of tRNAMet or acetyl-CoA, 2.5 μM of in vitro-transcribed tRNAMet or 200 μM acetyl-CoA was added to the reaction mixture. An aliquot (10 μl) of the reaction was quenched by formic acid at various time periods (7, 15, 23 and 30 s), spotted onto a PEI-cellulose TLC plate (Polygram Cel 300 PEI/UV254, Machery-Nagel) and developed with 0.75 M KH2PO4 (pH 3.5). The radioactivity was visualized and quantified by an imaging analyser (FLA-7000 system, Fujifilm, Japan) (Supplementary Figure S1). The initial velocity of each reaction was determined, and kinetic parameters were calculated by a Hanes–Woolf plot.
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to Takeo Suzuki (University of Tokyo), A Soma, Y Sekine (Rikkyo University) and J Kato (Tokyo Metropolitan University) for providing materials and technical assistance. Special thanks are due to Sarin Chimnaronk and Isao Tanaka (Hokkaido Univ) for communicating their unpublished results and for helpful suggestions. This work was supported by grants-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan (to TS); by a JSPS Fellowship for Japanese Junior Scientists (to YI); and by a grant from the New Energy and Industrial Technology Development Organization (NEDO) (to TS).
References
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM (2006) Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96 [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Xu J, Johnson RC, Schimmel P, de Crecy-Lagard V (2002) Identification of the tRNA-dihydrouridine synthase family. J Biol Chem 277: 25090–25095 [DOI] [PubMed] [Google Scholar]
- Bjork GR (1995) Biosynthesis and function of modified nucleosides. In tRNA: Structure, Biosynthesis, and Function, Söll DR, RajBhandary UL (eds), pp 165–205, Washington, DC: American Society for Microbiology [Google Scholar]
- Bochner BR, Ames BN (1982) Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem 257: 9759–9769 [PubMed] [Google Scholar]
- Bruenger E, Kowalak JA, Kuchino Y, McCloskey JA, Mizushima H, Stetter KO, Crain PF (1993) 5S rRNA modification in the hyperthermophilic archaea Sulfolobus solfataricus and Pyrodictium occultum. FASEB J 7: 196–200 [DOI] [PubMed] [Google Scholar]
- Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM (2008) Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′–3′ exonucleases Rat1 and Xrn1. Genes Dev 22: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JF (1998) Modified Nucleosides in Translation. Washington, DC: ASM press [Google Scholar]
- Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunin-Horkawicz S, Czerwoniec A, Gajda MJ, Feder M, Grosjean H, Bujnicki JM (2006) MODOMICS: a database of RNA modification pathways. Nucleic Acids Res 34: D145–D149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand JM, Bjork GR, Kuwae A, Yoshikawa M, Sasakawa C (1997) The modified nucleoside 2-methylthio-N6-isopentenyladenosine in tRNA of Shigella flexneri is required for expression of virulence genes. J Bacteriol 179: 5777–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand JM, Okada N, Tobe T, Watarai M, Fukuda I, Suzuki T, Nakata N, Komatsu K, Yoshikawa M, Sasakawa C (1994) vacC, a virulence-associated chromosomal locus of Shigella flexneri, is homologous to tgt, a gene encoding tRNA-guanine transglycosylase (Tgt) of Escherichia coli K-12. J Bacteriol 176: 4627–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Mistry J, Schuster-Bockler B, Griffiths-Jones S, Hollich V, Lassmann T, Moxon S, Marshall M, Khanna A, Durbin R, Eddy SR, Sonnhammer EL, Bateman A (2006) Pfam: clans, web tools and services. Nucleic Acids Res 34: D247–D251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H (2005) Modification and Editing of RNA: Historical Overview and Important Facts To Remember. Springer-Verlag: NY [Google Scholar]
- Gupta R (1984) Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem 259: 9461–9471 [PubMed] [Google Scholar]
- Hashimoto M, Ichimura T, Mizoguchi H, Tanaka K, Fujimitsu K, Keyamura K, Ote T, Yamakawa T, Yamazaki Y, Mori H, Katayama T, Kato J (2005) Cell size and nucleoid organization of engineered Escherichia coli cells with a reduced genome. Mol Microbiol 55: 137–149 [DOI] [PubMed] [Google Scholar]
- Ikeuchi Y, Shigi N, Kato J, Nishimura A, Suzuki T (2006) Mechanistic insights into sulfur-relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol Cell 21: 97–108 [DOI] [PubMed] [Google Scholar]
- Ikeuchi Y, Soma A, Ote T, Kato J, Sekine Y, Suzuki T (2005) Molecular mechamism of lysidine synthesis that determines tRNA identity and codon recognition. Mol Cell 19: 235–246 [DOI] [PubMed] [Google Scholar]
- Johansson MJ, Byström AS (2004) The Saccharomyces cerevisiae TAN1 gene is required for N4-acetylcytidine formation in tRNA. RNA 10: 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai G, Hashizume T, Miyazawa T, McCloskey JA, Yokoyama S (1989) Conformational characteristics of 4-acetylcytidine found in tRNA. Nucleic Acids Symp Ser 21: 61–62 [PubMed] [Google Scholar]
- Lemke CT, Howell PL (2001) The 1.6 A crystal structure of E. coli argininosuccinate synthetase suggests a conformational change during catalysis. Structure 9: 1153–1164 [DOI] [PubMed] [Google Scholar]
- McCarroll R, Olsen GJ, Stahl YD, Woese CR, Sogin ML (1983) Nucleotide sequence of the Dictyostelium discoideum small-subunit ribosomal ribonucleic acid inferred from the gene sequence: evolutionary implications. Biochemistry 22: 5858–5868 [Google Scholar]
- Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S (1988) Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336: 179–181 [DOI] [PubMed] [Google Scholar]
- Noma A, Kirino Y, Ikeuchi Y, Suzuki T (2006) Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J 25: 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A, Suzuki T (2006) Ribonucleome analysis identified enzyme genes responsible for wybutosine synthesis. Nucleic Acids Symp Ser 50: 65–66 [DOI] [PubMed] [Google Scholar]
- Numata T, Ikeuchi Y, Fukai S, Suzuki T, Nureki O (2006) Snapshots of tRNA sulphuration via an adenylated intermediate. Nature 442: 419–424 [DOI] [PubMed] [Google Scholar]
- Oashi Z, Murao K, Yahagi T, Von Minden DL, McCloskey JA, Nishimura S (1972) Characterization of C + located in the first position of the anticodon of Escherichia coli tRNA Met as N 4 -acetylcytidine. Biochim Biophys Acta 262: 209–213 [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ (2001) AAA+ superfamily ATPases: common structure—diverse function. Genes Cells 6: 575–597 [DOI] [PubMed] [Google Scholar]
- Rizzi M, Nessi C, Mattevi A, Coda A, Bolognesi M, Galizzi A (1996) Crystal structure of NH3-dependent NAD+ synthetase from Bacillus subtilis. EMBO J 15: 5125–5134 [PMC free article] [PubMed] [Google Scholar]
- Rozenski J, Crain PF, McCloskey JA (1999) The RNA modification database: 1999 update. Nucleic Acids Res 27: 196–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JR, Uhlenbeck OC (1998) BIochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci USA 85: 1033–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M, Sibbald PR, Wittinghofer A (1990) The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci 15: 430–434 [DOI] [PubMed] [Google Scholar]
- Soma A, Ikeuchi Y, Kanemasa S, Kobayashi K, Ogasawara N, Ote T, Kato J, Watanabe K, Sekine Y, Suzuki T (2003) An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol Cell 12: 689–698 [DOI] [PubMed] [Google Scholar]
- Sprinzl M, Vassilenko KS (2005) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 33: D139–D140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L, Schulman LH (1978) The role of the minor base N4-acetylcytidine in the function of the Escherichia coli noninitiator methionine transfer RNA. J Biol Chem 253: 6132–6139 [PubMed] [Google Scholar]
- Suzuki T (2005) Biosynthesis and function of tRNA wobble modifications. In Fine-Tuning of RNA Functions by Modification and Editing, Grosjean H (ed), Vol. 12, pp 24–69. New York: Springer-Verlag [Google Scholar]
- Suzuki T, Ikeuchi Y, Noma A, Suzuki T, Sakaguchi Y (2007) Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol 425: 211–229 [DOI] [PubMed] [Google Scholar]
- Takano Y, Takayanagi N, Hori H, Ikeuchi Y, Suzuki T, Kimura A, Okuno T (2006) A gene involved in modifying transfer RNA is required for fungal pathogenicity and stress tolerance of Colletotrichum lagenarium. Mol Microbiol 60: 81–92 [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Natale DA, Garkavtsev IV, Tatusova TA, Shankavaram UT, Rao BS, Kiryutin B, Galperin MY, Fedorova ND, Koonin EV (2001) The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res 29: 22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Suzuki T, Hanada T, Watanabe K (2004) Functional compatibility of elongation factors between mammalian mitochondrial and bacterial ribosomes: characterization of GTPase activity and translation elongation by hybrid ribosomes bearing heterologous L7/12 proteins. J Mol Biol 336: 331–342 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Klem TJ, Deras ML, Davisson VJ, Smith JL (1996) The crystal structure of GMP synthetase reveals a novel catalytic triad and is a structural paradigm for two enzyme families. Nat Struct Biol 3: 74–86 [DOI] [PubMed] [Google Scholar]
- Thomas G, Gordon J, Rogg H (1978) N4-acetylcytidine. A previously unidentified labile component of the small subunit of eukaryotic ribosomes. J Biol Chem 253: 1101–1105 [PubMed] [Google Scholar]
- Wang X, Jia H, Jankowsky E, Anderson JT (2008) Degradation of hypomodified tRNA(iMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA 14: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Lauring B (2007) AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic 8: 1657–1667 [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Nishimura S (1995) Modified Nucleosides and Codon Recognition. Washington, DC: ASM Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information