Abstract
Two expert research microscopists, each blinded to the other’s reports, diagnosed single-species malaria infections in 2,141 adults presenting at outpatient malaria clinics in Tak Province, Thailand, and Iquitos, Peru, in May–August 1998, May–July 1999, and May–June 2001. Plasmodium vivax patients with gametocytemia had higher fever and higher parasitemia than those without gametocytemia; temperature correlated with parasitemia in the patients with gametocytemia. Plasmodium falciparum patients with gametocytemia had lower fever than those without gametocytemia, but similar parasitemia; temperature correlated with parasitemia in the patients without gametocytemia. Hematologic data in Thailand in 2001 showed lower platelet counts in P. vivax patients with gametocytemia than in the P. vivax patients without gametocytemia, whereas P. falciparum patients with gametocytemia had similar platelet counts but lower red blood cell counts, hemoglobin levels, hematocrit levels, and higher lymphocyte counts than patients without gametocytemia.
Gametocytes are the sexual, nonreplicating blood-stage forms of Plasmodium spp. that are transmission agents to mosquitoes. They are products of the asexual, replicating, nontransmissible forms with which the pathology of malaria is associated. Gametocytes themselves are clinically benign, but their production represents a diversion of parasites from the geometric growth of asexual forms; each asexual form that becomes a gametocyte might instead become millions of asexual forms (Carter and Graves, 1988; McKenzie and Bossert, 1998; Talman et al., 2004). Thus, the higher the rate of gametocyte production earlier in an infection, the longer it is until parasitemia reaches a pyrogenic level or any other given density.
Gametocytes attract relatively little research attention, although classic studies of adult malaria cases reported positive relationships between gametocytemia, mildness of symptoms, leukocyte proliferation, and age (Thomson, 1911), and higher gametocyte-to-asexual form ratios in patients who cleared infections without treatment than in those requiring drugs (Kitchen and Putnam, 1942). Recent work has focused on children in sub-Saharan Africa infected with P. falciparum in whom gametocytemia is associated with an absence of fever (von Seidlein et al., 2001; Sowunmi et al., 2004), low (von Seidlein et al., 2001; Sowunmi et al., 2004) or high (Githeko et al., 1992; Drakeley et al., 1999; Bousema et al., 2004) asexual parasitemia, and either anemia (Drakeley et al., 1999; von Seidlein et al., 2001) or its absence (Sowunmi et al., 2004).
Few previous studies have examined characteristics associated with gametocytemia in adults, or in endemic regions other than Africa, or in species other than P. falciparum. Here, we report on gametocytemia in adults visiting local outpatient malaria clinics in western Thailand and northern Peru, where P. vivax and P. falciparum are coendemic.
MATERIALS AND METHODS
Data were collected during studies of malaria rapid diagnostic devices, for which the methods are fully described elsewhere (Forney et al., 2001, 2003; McKenzie et al., 2003, 2005, 2006; Erhart et al., 2004; O’Meara et al., 2005). Briefly, participants presented on their own initiative to existing outpatient malaria clinics operated by local public health authorities at Maesod, Tak Province, Thailand, or Iquitos, Peru, 28 May–28 August 1998 or 17 May–9 July 1999 (age ≥15 yr, Thailand; or ≥1 yr, Peru), with fever (oral temperature ≥38 C), headache, or self-reported history of fever within the previous 72 hr. Severely ill patients were immediately referred to district hospitals, and not enrolled. During the study, clinic staff retained full responsibility for patient care; diagnostic and treatment decisions were made independent of the study protocol. The same criteria and protocol held in the 2001 study, which was conducted 18 May–29 June, in the Mae Ku, Maesod, and Phob Phra Districts of Tak Province, Thailand, and enrolled patients ≥20 yr of age. The protocol was reviewed by the Institutional Review Board, Walter Reed Army Institute of Research, and the Human Subjects Safety Review Board, U.S. Army Medical Research and Materiel Command. It was reviewed, approved, and conducted with oversight by the Thai Ministry of Public Health and the Peruvian Ministry of Health.
At enrollment, patients were asked how long they had felt ill; responses were recorded as 1–8 days, with 8 representing >7 days. They were also asked whether they had taken antimalarial drugs within the previous 2 wk; this analysis excludes the 25 enrollees who gave positive responses. Males constituted 66–70% of participants in Thailand and 56% of participants in Peru. No patients <18 yr old presented in 1999; this analysis removes the 115 enrollees <18 yr old from the 1998 data, rendering the 1998 and 1999 age distributions indistinguishable (Mann-Whitney U-test; P = 0.07 for Thailand; P = 0.10 for Peru). Although the median age of participants in Thailand was 25 in each year, the 2001 age distribution differed from 1998 and 1999 (P < 0.001), reflecting the absence of 18- to 19-yr-olds. In Peru, the median age of participants was 33 in 1998 and 31 in 1999. In Thailand, participant males were 1 to 2 yr older than females; in Peru, they were 1 to 3 yr younger.
The oral temperature of each patient was taken immediately before blood was drawn. Approximately 2 ml of venous blood was drawn from each individual into an EDTA-filled tube for blood films and an automated blood count. Precise volumes of well-mixed whole blood were micropipetted and prepared as thick and thin smears on each of 3 pre-cleaned slides by well-trained technicians following standardized procedures. One slide was used promptly by clinic staff for diagnosis and medical intervention if indicated. The remaining 2 slides were held overnight, then stained with 3% Giemsa. Each of 2 expert microscopists, blinded to the other’s interpretation, read the same slides. Two hundred oil-immersion high-power fields on the thick film were read before any slide was interpreted as negative; the thin film was used only for species determination. Species identifications and density estimates were made solely on the basis of asexual blood forms. If after 200 white blood cells (WBCs) were enumerated 10 or more asexual parasites had been counted, the total number was recorded. If asexual parasites were present but they numbered fewer than 10 per 200 WBCs, the microscopists continued examining the smear, recording parasites until 500 WBCs had been counted.
Blood tubes were promptly transported on ice to a field laboratory, where in Thailand, cell counts were performed using a Coulter automated cell counter (Beckman-Coulter, Inc., Fullerton, California), or in Peru, a QBC centrifugal hematology system (Becton-Dickinson Diagnostic Systems, Sparks, Maryland). The extensive quality assurance procedures are described by Forney et al. (2001). WBC counts had skewed distributions, as expected (Bain, 2002). Because differences between the Coulter and QBC systems may have confounded analyses of reported densities (McKenzie et al., 2005), we did not pursue comparisons between sites. Detailed hematologic data were collected only in 2001 (Erhart et al., 2004). We used the Mann-Whitney U-test and Spearman’s rank correlation coefficient to compare distributions (Sokal and Rohlf 1981), and give P values for 2-tailed tests.
The 1,043 P. vivax and 1,098 P. falciparum cases analyzed here exclude 3,745 cases for whom the 2 microscopists agreed on the absence of blood stage Plasmodium spp., 482 for whom they disagreed on the presence or species of asexual parasites or the presence of gametocytes, 80 for whom one or both reported a mixed-species infection, and 9 with gametocyte-only infections (4 P. vivax and 5 P. falciparum). In no report were asexual forms and gametocytes of different species. Plasmodium falciparum gametocytes were recorded at both sites, but P. vivax gametocytes were recorded only in Thailand; therefore, our calculations for P. vivax and comparisons between species include only data from Thailand. The asexual form and gametocyte densities used here are the average of the 2 microscopists’ reports.
RESULTS
The age of patients with and without gametocytemia differed only among P. vivax patients in Thailand, in 1998: the patients with gametocytemia were younger (median age, 23 vs. 25; Mann-Whitney U-test, P = 0.017). The number of days the patients reported having felt ill differed between patients with and without gametocytemia only among P. falciparum patients in Thailand, in 1999 (median, 5 vs. 3 days; P = 0.000008) and among P. vivax patients in Thailand, in 2001 (3 vs. 2 days; P = 0.005). Gametocyte density did not correlate with age or reported number of days ill in any of the 8 site-year-species combinations (Spearman, P > 0.05).
Gametocytemia was much more frequent in P. vivax than P. falciparum infections (57% vs. 9%), but the species did not differ in gametocyte density (median 151 vs. 132 per microliter; P > 0.06) or the ratio of gametocytes to asexual forms (median, 0.025 vs. 0.037; P > 0.12). Gametocyte density correlated with asexual parasitemia (P < 0.00000002) and WBC count (P < 0.002) in P. vivax, but not P. falciparum infections (P > 0.06), and correlated with patient temperature only in the 2001 P. vivax infections (P < 0.00000001). Parasitemia and fever were lower in P. vivax than P. falciparum among patients without gametocytemia (Table I), but not among patients with gametocytemia (P > 0.05).
Table I.
Median values (95% confidence limits) for per-microliter gametocyte densities, per-microliter asexual parasite densities, and patient temperatures in patients with and without gametocytemia infected with P. vivax and P. falciparum.
| Gametocytemia (log 10) | Parasitemia (log 10) | Temperature (C) | |
|---|---|---|---|
| P. vivax | |||
| Thailand 1998 | 2.37 (2.26–2.51) | 3.76 (3.65–3.89)
2.26 (1.76–2.60)* |
38.2 (37.8–38.7)
37.7 (37.5–37.9)* |
| Thailand 1999 | 2.24 (2.14–2.35) | 3.79 (3.62–3.98)
2.57 (2.20–2.86)* |
38.3 (37.7–38.9)
37.5 (37.2–37.8)* |
| Thailand 2001 | 2.04 (1.95–2.12) | 3.91 (3.78–4.01)
2.80 (2.61–2.99)* |
38.4 (38.0–38.8)
37.5 (37.3–37.9)* |
| P. falciparum | |||
| Thailand 1998 | 2.27 (1.73–2.70) | 3.58 (2.42–4.10)
4.00 (3.82–4.14)* |
37.8 (37.2–38.7)
38.2 (37.9–38.8)* |
| Thailand 1999 | 2.03 (1.58–2.51) | 4.01 (2.89–4.36)
3.96 (3.65–4.20)* |
37.6 (37.1–38.1)
38.2 (37.9–38.7)* |
| Thailand 2001 | 2.00 (1.49–2.64) | 3.69 (2.29–4.70)
4.02 (3.89–4.23)* |
37.6 (36.6–38.7)
38.7 (38.4–38.9)* |
| Peru 1998 | 2.43 (1.84–3.22) | 3.67 (2.35–4.30)
4.14 (3.76–4.34) |
37.3 (36.0–38.3)
37.8 (37.6–38.4) |
| Peru 1999 | 1.74 (1.51–3.51) | 3.75 (2.41–5.34)
3.58 (3.07–4.12) |
37.1 (36.4–37.4)
38.0 (37.2–38.5) |
Mann-Whitney U-test, P < 0.00007 for comparisons between patients infected with P. vivax and P. falciparum.
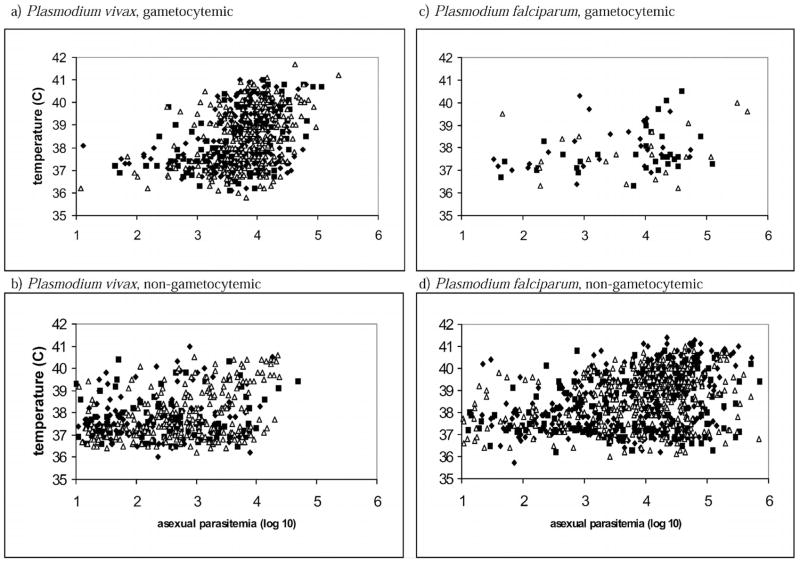
Gametocytemic P. vivax had higher fever and higher asexual parasitemia than those without gametocytemia (Fig. 1; Table I; all Mann-Whitney U-test, P < 0.0002). Temperature correlated with asexual parasitemia in gametocytemic P. vivax patients (Spearman, P < 0.0001), but not in patients without gametocytemia (P > 0.05) in 1998 and 1999, and in P. vivax patients with and without gametocytemia in 2001 (P < 0.0000001).
Figure 1.
Temperatures (C) of patients infected with Plasmodium vivax (left) or Plasmodium falciparum (right), gametocytemic (top) or nongametocytemic (bottom), in Thailand, in 1998 (◆), 1999 (■), and 2001 (△) plotted against asexual parasitemia (log 10).
Gametocytemic P. falciparum patients had lower fever than those without gametocytemia (Fig. 1; Table I; P < 0.020) except in Thailand, in 1998 (P = 0.11). Their asexual parasitemia was indistinguishable from those without gametocytemia in 1998 and 2001 (Table I; P > 0.58), but it was marginally lower in 1998 (P [for Thailand] = 0.025; P [for Peru] = 0.031). Temperature clearly correlated with parasitemia in P. falciparum patients without gametocytemia in 4 of the 5 site-yr combinations (P < 0.0005), and marginally so in Peru, in 1999 (P = 0.028). Temperature marginally correlated with parasitemia in gametocytemic P. falciparum patients in Thailand, in 1999 (P = 0.025), but clearly not in the other 4 site-year combinations (P > 0.59).
WBC counts did not differ between patients with and without gametocytemia infected with either species (Mann-Whitney U-test, P > 0.13). WBC count and asexual-form density correlated in gametocytemic P. vivax cases in all 3 yr (Spearman; P < 0.001) in nongametocytemic P. vivax cases in 2001 (P = 0.007), and in nongametocytemic P. falciparum cases in 1999 (P = 0.011).
Detailed hematologic data were collected on the patients in Thailand in 2001. In previous work, we reported differences between male and female participants that produce bimodal distributions in the patient populations (Erhart et al., 2004). The results shown here combine the sexes, but they also hold when the comparisons are made strictly among men or among women, with one exception. Plasmodium vivax gametocyte carriers and noncarriers did not differ in lymphocyte count in the overall population (P = 0.149), but they did when each sex was considered separately (males, 1.6 vs. 1.7, P = 0.029; females, 1.5 vs. 1.8, P = 0.011). Platelet counts in gametocytemic P. vivax patients were much lower than in P. vivax patients without gametocytemia (Table II). Platelet counts in gametocytemic P. falciparum patients were similar to those in the patients without gametocytemia (P = 0.36), but their red blood cell counts, hemoglobin levels, and hematocrits were lower, and their lymphocyte counts were higher (Table II). Gametocyte density in P. vivax infections correlated negatively with platelet counts (P < 0.005). Temperature correlated negatively with lymphocyte counts and platelet counts in P. vivax patients, regardless of gametocytemia (P < 0.01), and in P. falciparum patients without gametocytemia (P < 0.00006); parasitemia followed the same pattern (P < 0.0003), except with lymphocyte counts in gametocytemic P. vivax patients (P = 0.167).
Table II.
Median values (95% confidence limits) for platelet count, red blood cell count, hemoglobin level, hematocrit, lymphocyte count, and white blood cell count in patients infected with P. vivax and P. falciparum in Thailand, in 2001.*
|
P. vivax† |
P. falciparum† |
|||
|---|---|---|---|---|
| Gametocytemic | Nongametocytemic | Gametocytemic | Nongametocytemic | |
| PLT | 109 (93–119)* | 172.5 (153–189)* | 121 (47–267) | 114 (104–125) |
| RBC | 4.54 (4.43–4.64) | 4.75 (4.68–4.88) | 3.91 (3.4–4.25)* | 4.6 (4.5–4.72)* |
| HGB | 12.7 (12.5–13.0) | 13.4 (13.0–13.8) | 10.6 (8.1–13.1)* | 13.1 (12.7–13.4)* |
| HCT | 38.4 (37.4–39.2) | 40.4 (39.4–41.5) | 32.5 (25.2–37.7)* | 38.9 (38.1–40.2)* |
| LYM | 1.5 (1.4–1.7) | 1.75 (1.6–2.0) | 1.9 (1.3–2.6)* | 1.4 (1.3–1.5)* |
| WBC | 6.4 (6.0–6.9) | 6.3 (5.9–6.8) | 6.7 (4.5–8.5) | 6.1 (5.6–6.4) |
Note that the distributions do not separate males and females and so are typically bimodal (see text). PLT indicates platelet count (thousands/μl); RBC indicates red blood cells (millions/μl); HGB indicates hemoglobin (g/dl); HCT indicates hematocrit (%); LYM indicates lymphocytes (thousands/μl); WBC indicates white blood cells (thousands/μl).
Values with * denote Mann-Whitney U-test, P < 0.0004 for comparisons between patients with and without gametocytemia.
DISCUSSION
This large, multiyear, multisite, cross-sectional analysis of adults presenting at malaria clinics points to key characteristics of fever, asexual parasitemia, and hematology associated with gametocytemia in P. vivax and P. falciparum infections, and to key differences between the 2 species. In P. vivax infections, gametocytemia was associated with higher fever, higher asexual parasitemia, and lower platelet counts. Plasmodium vivax gametocyte density correlated with asexual parasitemia and WBC count, and in the gametocytemic infections, temperature and WBC count correlated with asexual parasitemia. In P. falciparum infections, gametocytemia was generally associated with lower fever, and with hematologic markers of anemia, but asexual parasitemia and platelet counts were similar to nongametocytemic infections; temperature correlated with asexual parasitemia in nongametocytemic infections.
Plasmodium vivax and P. falciparum infections differed dramatically in gametocyte prevalence, but not gametocyte density or gametocyte-to-asexual-form ratio. Temperature and asexual parasitemia were lower with P. vivax than P. falciparum among nongametocytemic but not gametocytemic infections. The asexual parasitemia “threshold” for fever is believed to be lower for P. vivax than P. falciparum (Boyd, 1938; Luxemberger et al., 1997; McKenzie et al., 2002), but to the best of our knowledge, gametocytemia has not previously been considered. Correlation between patient temperature and asexual parasitemia in our study populations generally depended on the presence of gametocytes in P. vivax infections, and on the absence of gametocytes in P. falciparum infections.
To the best of our knowledge, this is the first report on characteristics associated with gametocytemia at any South American site, but there have been several previous reports from Thailand. In a specialty hospital in Bangkok over a wide age range of patients, gametocytemia in P. falciparum infections was associated with higher asexual parasitemia, lower hemoglobin levels, and longer reported duration of symptoms, but not differences in age or temperature (Nacher et al., 2002). Gametocytemia in P. vivax infections was associated with higher asexual parasitemia, lower fevers, and lower red blood cell counts, but not with differences in age or the reported duration of symptoms (Nacher, Silachamroon et al., 2004). In the hospital, P. falciparum gametocyte density correlated with duration of symptoms, but not asexual-form density; P vivax gametocyte density correlated with asexual parasitemia, and inversely with temperature, but not duration of symptoms. In a clinic in the Shoklo refugee camp in northwest Thailand, with a study population largely younger than age 14, gametocytemia in P. falciparum infections was associated with lower asexual parasitemia, absence of fever, and lower hematocrit, but not differences in age or reported duration of symptoms (Price et al., 1999). In P. vivax infections, gametocytemia was associated with younger age (Nacher, Carrara et al., 2004), but no information was given on other variables considered in our study.
Our populations more closely resembled the 2 hospital studies with respect to age distribution, and the camp study with respect to clinical symptoms other than temperature. The clear differences between our results for P. vivax infections and those from the hospital are in fever and red blood cell count, which we cannot explain. Our results for P. falciparum infections resemble those from the camp more than the hospital. Gametocytemic P. falciparum infections in the hospitalized patients had developed longer, had greater asexual parasitemia, higher fevers, and more severe symptoms; “severe malaria” was cited as a risk factor for gametocytemia. In our study, cases deemed “severe” were immediately treated and not enrolled.
Some of our results seem to reflect a plausible timeline of events within individual infections, i.e., fever and parasitemia may be higher in patients with P. vivax infection with gametocytemia than in those without gametocytemia because P. vivax gametocytes are in circulation as soon as they are produced and reach detectable levels as parasitemia and fever reach high levels. However, with P. falciparum, fever may be lower in patients with gametocytemia than in those without gametocytemia because P. falciparum gametocytes enter the circulation only after 8–10 days of sequestration (Kitchen and Putnam, 1942; Carter and Graves, 1988), and so reach detectable levels after fever drops from peak levels. Other aspects are puzzling; for instance, why there were so few differences between patients with gametocytemia and those without gametocytemia in the number of days reported ill, or why only half of the gametocytemia P. falciparum patients reported >3 days ill.
Further, it may be that the association of higher fever, higher parasitemia, and detectable gametocytemia in these adult P. vivax patients reflects effective immune response as much as clinical severity per se. It has been reported that P. vivax gametocytes are killed by pyrogenic cytokines and complementary factors in the paroxysm plasma of nonimmune patients, but that these factors and their effects are suppressed with the development of clinical immunity (Karunaweera et al., 1992). For P. falciparum, it has been reported that specific immune suppression of gametocytes develops with repeated exposure (Baird et al., 1991), but that seasonal fluctuations in gametocyte prevalence are greater among adults than children (Rosenberg et al., 1990). Detailed longitudinal studies should investigate the conjunction of parasitological, immunological, and clinical characteristics associated with gametocytemia.
Acknowledgments
We thank the AFRIMS field study teams for their dedication and technical assistance, and gratefully acknowledge the contributions of J. Adams, W. Bossert, S. Hanna, H. Metros, and 2 anonymous reviewers. This work was supported by the U.S. Army Medical Research and Materiel Command. The views presented are those of the authors and do not purport to represent those of our respective institutions.
Contributor Information
F. Ellis McKenzie, Fogarty International Center, National Institutes of Health, Bethesda, Maryland 20892, e-mail: em225k@nih.gov.
Chansuda Wongsrichanalai, U.S. Naval Medical Research Unit No. 2 (NAMRU-2), Jakarta, Indonesia..
Alan J. Magill, Walter Reed Army Institute of Research, Silver Spring, Maryland.
J. Russ Forney, Walter Reed Army Institute of Research, Silver Spring, Maryland..
Barnyen Permpanich, Armed Forces Research Institute of Medical Sciences (AFRIMS), Bangkok, Thailand..
Carmen Lucas, U.S. Naval Medical Research Center Detachment (NMRCD), Lima, Peru..
Laura M. Erhart, Armed Forces Research Institute of Medical Sciences (AFRIMS), Bangkok, Thailand.
Wendy P. O’Meara, Fogarty International Center, National Institutes of Health, Bethesda, Maryland 20892
David L. Smith, Fogarty International Center, National Institutes of Health, Bethesda, Maryland 20892
Jeeraphat Sirichaisinthop, Department of Communicable Disease Control, Ministry of Public Health, Bangkok, Thailand..
Robert A. Gasser, Jr., Walter Reed Army Institute of Research, Silver Spring, Maryland.
LITERATURE CITED
- Bain BJ. Blood cells. Blackwell Science; Oxford, U.K.: 2002. p. 408. [Google Scholar]
- Baird JK, Jones TR, Purnomo, Masbar S, Ratiwayanto S, Leksana B. Evidence for specific suppression of gametocytemia by Plasmodium falciparum in residents of hyperendemic Irian Jaya. American Journal of Tropical Medicine and Hygiene. 1991;44:183–190. doi: 10.4269/ajtmh.1991.44.183. [DOI] [PubMed] [Google Scholar]
- Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malaria Journal. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd MF. The threshold of parasite density in relation to clinical activity in primary infections with Plasmodium vivax. American Journal of Tropical Medicine. 1938;18:497–503. [Google Scholar]
- Carter R, Graves PM. Gametocytes. In: Wernsdorfer WH, McGregor I, editors. Malaria. Churchill Livingstone; Edinburgh, U.K.: 1988. pp. 253–306. [Google Scholar]
- Drakeley CJ, Secka I, Correa S, Greenwood BM, Targett GA. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Tropical Medicine and International Health. 1999;4:131–138. doi: 10.1046/j.1365-3156.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Erhart LM, Yingyuen K, Chuanak N, Buathong N, Laoboonchai A, Miller RS, Meshnick SR, Gasser RA, Jr, Wongsrichanalai C. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. American Journal of Tropical Medicine and Hygiene. 2004;70:8–14. [PubMed] [Google Scholar]
- Forney JR, Magill AJ, Wongsrichanalai C, Sirichaisinthop J, Bautista CT, Heppner DG, Miller RS, Ockenhouse CF, Gubanov A, Shafer R, DeWitt CC, Quino-Ascurra HA, Kester KE, Kain KC, Walsh DS, Ballou WR, Gasser RA., Jr Malaria rapid diagnostic devices: Performance characteristics of the ParaSight F device determined in a multisite field study. Journal of Clinical Microbiology. 2001;39:2884–2890. doi: 10.1128/JCM.39.8.2884-2890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney JR, Wongsrichanalai C, Magill AJ, Craig LG, Sirichaisinthop J, Bautista CT, Miller RS, Ockenhouse CF, Kester KE, Aronson NE, Andersen EM, Quino-Ascurra HA, Vidal C, Moran KA, Murray CK, DeWitt CC, Heppner DG, Kain KC, Ballou WR, Gasser RA., Jr Devices for rapid diagnosis of malaria: Evaluation of prototype assays that detect Plasmodium falciparum histidine-rich protein 2 and a Plasmodium vivax-specific antigen. Journal of Clinical Microbiology. 2003;41:2358–2366. doi: 10.1128/JCM.41.6.2358-2366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH. The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:355–358. doi: 10.1016/0035-9203(92)90216-y. [DOI] [PubMed] [Google Scholar]
- Karunaweera ND, Carter R, Grau GE, Kwiatkowski D, Del Giudice G, Mendis KN. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clinical and Experimental Immunology. 1992;88:499–505. doi: 10.1111/j.1365-2249.1992.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen SF, Putnam P. Observations of the mechanism of the parasite cycle in falciparum malaria. American Journal of Tropical Medicine. 1942;22:361–386. [Google Scholar]
- Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91:256–62. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. A target for intervention in Plasmodium falciparum infections. American Journal of Tropical Medicine and Hygiene. 1998;58:763–767. doi: 10.4269/ajtmh.1998.58.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Jeffery GM, Collins WE. Plasmodium vivax blood-stage dynamics. Journal of Parasitology. 2002;88:521–535. doi: 10.1645/0022-3395(2002)088[0521:PVBSD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Prudhomme WA, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Jr, Wongsrichanalai C. White blood cell counts and malaria. Journal of Infectious Diseases. 2005;192:323–330. doi: 10.1086/431152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Sirichaisinthop J, Miller RS, Gasser RA, Jr, Wongsrichanalai C. Dependence of malaria detection and species diagnosis by microscopy on parasite density. American Journal of Tropical Medicine and Hygiene. 2003;69:372–376. [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Smith DL, O’Meara WP, Forney JR, Magill AJ, Permpanich B, Erhart LM, Sirichaisinthop J, Wongsrichanalai C, Gasser RA., Jr Fever in mixed-species malaria. Clinical Infectious Diseases. 2006;42:1713–1718. doi: 10.1086/504330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher M, V, Carrara I, McGready R, Ashley E, Nguen JV, Thwai KL, Looareesuwan S, Nosten F. Seasonal fluctuations in the carriage of Plasmodium vivax gametocytes in Thailand. Annals of Tropical Medicine and Parasitology. 2004;98:115–120. doi: 10.1179/000349804225003145. [DOI] [PubMed] [Google Scholar]
- Nacher M, Silachamroon U, Singhasivanon P, Wilairatana P, Phumratanaprapin W, Fontanet A, Looareesuwan S. Risk factors for Plasmodium vivax gametocyte carriage in Thailand. American Journal of Tropical Medicine and Hygiene. 2004;71:693–695. [PubMed] [Google Scholar]
- Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Tosukhowong T, Vannaphan S, Gay F, Mazier D, Looareesuwan S. Decreased hemoglobin concentrations, hyperparasitemia, and severe malaria are associated with increased Plasmodium falciparum gametocyte carriage. Journal of Parasitology. 2002;88:97–101. doi: 10.1645/0022-3395(2002)088[0097:DHCHAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- O’Meara WP, McKenzie FE, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Jr, Wongsrichanalai C. Sources of variability in determining malaria parasite density by microscopy. American Journal of Tropical Medicine and Hygiene. 2005;72:593–598. [PMC free article] [PubMed] [Google Scholar]
- Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, ter Kuile F, van Vugt M, Chongsuphajaisiddhi T, White NJ. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. American Journal of Tropical Medicine and Hygiene. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Andre RG, Ketrangsee S. Seasonal fluctuation of Plasmodium falciparum gametocytaemia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:29–33. doi: 10.1016/0035-9203(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. W. H. Freeman; New York, New York: 1981. p. 859. [Google Scholar]
- Sowunmi A, Fateye BA, Adedeji AA, Fehintola FA, Happi TC. Risk factors for gametocyte carriage in uncomplicated falciparum malaria in children. Parasitology. 2004;129:255–262. doi: 10.1017/s0031182004005669. [DOI] [PubMed] [Google Scholar]
- Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: The puberty of Plasmodium falciparum. Malaria Journal. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. A research into the production, life and death of crescents in malignant tertian malaria, in treated and untreated cases, by an enumerative method. Annals of Tropical Medicine and Parasitology. 1911;5:57–82. [Google Scholar]
- von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. American Journal of Tropical Medicine and Hygiene. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]