Abstract
We have used a relatively simple but accurate model for predicting the impact of integrated transmission control on the malaria entomologic inoculation rate (EIR) at four endemic sites from across sub-Saharan Africa and the southwest Pacific. The simulated campaign incorporated modestly effective vaccine coverage, bed net use, and larval control. The results indicate that such campaigns would reduce EIRs at all four sites by 30- to 50-fold. Even without the vaccine, 15- to 25-fold reductions of EIR were predicted, implying that integrated control with a few modestly effective tools can meaningfully reduce malaria transmission in a range of endemic settings. The model accurately predicts the effects of bed nets and indoor spraying and demonstrates that they are the most effective tools available for reducing EIR. However, the impact of domestic adult vector control is amplified by measures for reducing the rate of emergence of vectors or the level of infectiousness of the human reservoir. We conclude that available tools, including currently neglected methods for larval control, can reduce malaria transmission intensity enough to alleviate mortality. Integrated control programs should be implemented to the fullest extent possible, even in areas of intense transmission, using simple models as decision-making tools. However, we also conclude that to eliminate malaria in many areas of intense transmission is beyond the scope of methods which developing nations can currently afford. New, cost-effective, practical tools are needed if malaria is ever to be eliminated from highly endemic areas.
INTRODUCTION
Malaria transmission intensity is best expressed as the entomologic inoculation rate (EIR) which directly reflects the exposure of humans to pathogenic Plasmodium parasites.1–3 In malaria-endemic parts of sub-Saharan Africa and the southwest Pacific, transmission intensity can vary from undetectable levels to more than 1,000 infective bites per year.1,2 Entomologic inoculation rate levels of one infective bite per year, or less, can readily sustain prevalence in excess of 40% for Plasmodium falciparum, the most pathogenic of the species of human malaria parasites.2 Although the clinical outcome of malaria infection depends on the specific immunological and pathological interactions of parasite strains and individual humans,4–9 most, if not all, strains of P. falciparum are potentially life-threatening.10,11 Stable, endemic malaria, at any level of transmission, is inevitably associated with a heavy disease burden.2,12–14 Clearly, very large and sustainable reductions of transmission intensity would be required to negate the public health impact of malaria in endemic areas of sub-Saharan Africa and the southwest Pacific. Nevertheless, despite considerable recent debate,15–18 the balance of existing evidence strongly supports the view that effective malaria transmission-control interventions19,20 will always be beneficial with respect to all-cause mortality.2,12,14,20 Malaria transmission control can reduce morbidity and mortality,12,14,20 but large reductions in EIR are required in most endemic settings.2,12,14,20
Transmission control and clinical treatment are separate components of any malaria control program and should be treated as such.2,11 Rapid and appropriate treatment of clinical cases can drastically reduce the number of deaths resulting from malaria but cannot reduce the incidence of attacks and has a limited impact on morbidity.11,21 What is not clear is whether wide-scale chemoprophylaxis reduces or increases the infectiousness of the human reservoir in endemic areas.22–25 Even if the infectiousness of the human reservoir can be reduced, it is difficult to justify the use of antimalarial drugs for transmission control. This is because the widespread administration of drugs is not only expensive and difficult to implement, but also promotes resistance in the parasite population and makes life-threatening cases more difficult to treat.26 Clearly we should not compromise the safety net of effective treatment and cure for the sake of partial prevention. Therefore, drugs should not be used as a transmission control tool in endemic areas although they are useful for controlling epidemics in areas of unstable transmission.27 Furthermore, recent modeling studies have suggested that the reversal of these roles may be useful: suppression of transmission intensity may help slow the evolution of drug resistance.28
Entomologic inoculation rate can be estimated as the product of the human reservoir infectiousness (κ), the lifetime transmission potential of individual mosquitoes (L) and the rate at which they emerge from larval breeding sites (E) relative to human population size (E/Nh):1
| (1) |
The structure of this equation directly implies that measures which reduce any of these contributors will amplify each other’s effects when combined. These three contributors are also discrete targets for transmission control that are reduced by quite different interventions. The only intervention we can envisage which could usefully reduce κ, and which is likely to be available in the foreseeable future is a malaria vaccine.29 Tools for the reduction of L include indoor spraying, bed nets, and zooprophylaxis12,19,20 whereas source reduction and other forms of larval control represent well-established methods for reducing E/Nh.30–32
Here we estimate the potential impact of these measures on EIR and assess their potential contributions to integrated transmission control in highly endemic areas. The impact of generalized control programs on EIR is estimated at four malaria-endemic sites in Nigeria, Tanzania, and Papua New Guinea for which site-specific predictors of EIR have been reported.1 Overall, we conclude that existing tools can reduce malaria transmission intensity enough to considerably reduce mortality, even in highly endemic areas, and should be applied in a concerted manner as components of integrated transmission control programs. However, the model also predicts that the elimination of malaria in areas of intense transmission is beyond the scope of methods that are available and affordable in developing nations. New tools are needed if this goal is ever to be achieved.
METHODS
Modeling malaria transmission intensity at four endemic sites
All terminology and abbreviations are consistent with Table 1 of the companion paper, which describes the model.1 This model has been tested using published data from four P. falciparum-endemic sites where values for EIR, the sporozoite rate (S), human biting rate (HBt), human reservoir infectiousness (κ), human blood-feeding preference (Q), survival rate per feeding cycle (Pf) and sporogonic incubation period (n) have all either been measured directly or calculated where reported values for other parameters allow it.1 For details of these parameters, see Table 2 of the accompanying paper.1 The model predicted EIR values for the four sites, which were 1.17 ± 0.37 (range 0.84–1.59) times those measured in the field and all outputs were consistent with field measurements and previous models.1 The four sites, namely Kankiya and Kaduna in Nigeria, Namawala in Tanzania, and Butelgut in Papua New Guinea are foci of intense P. falciparum transmission, morbidity, and mortality.1 These sites span a ten-fold range of EIR and a wide range of environmental conditions from across the African continent and the southwest Pacific.1 Briefly, Kankiya33 and Kaduna34,35 are in the dry Savannah region of Nigeria; Namawala is near the flood plain of the Kilombero River in Tanzania36–38 and Butelgut is in the forested foothills of Madang Province in Papua New Guinea.39–41 For simplicity, our model estimates EIR for the predominant vector species at each site, which are Anopheles arabiensis at Kankiya, Anopheles gambiae sensu lato, at Kaduna and Namawala, and Anopheles punctulatus at Butelgut.1 In order to calculate the impact of the combined effects of the intervention on the human biting rate (Hbt) it is calculated from the proposed reductions in vector emergence rate (E) and mean number of human bites per lifetime (bh) similar to equation 12 in the original model for calculating EIR:1
| (2) |
Modeling the impact of integrated transmission control on EIR
We propose the following set of feasible transmission control measures and model their combined impact on EIR by modeling their effects on κ(human resevoir infectionsness), Q (human feeding preference of the vector), Pf (survival per feeding cycle by the vector), and E/Nh based on literature estimates of their potential impact. All assumptions and input values for baseline conditions (no transmission control) are as described in Tables 2 and 3 of the accompanying paper.1 For each parameter that a given control measure could be expected to affect, a reasonable proportional impact was chosen, based on published values and reasonable guesses. These values were chosen and fixed a priori so that the results of the model would not be biased to fit predicted values for outcome variables to those reported in the literature. Initially, we predicted the impact of a program based on only insecticide-treated bed nets. Then the effects of a more integrated program incorporating a malaria vaccine and larval control were predicted.
Modeling the impact of insecticide-treated bed nets
Cost-effective tools that reduce Q or Pf or that increase f, the length of the feeding cycle, include indoor spraying33,42,43 and bed-net impregnation43–47 with insecticides. Bed nets have been reported to reduce vector survival per feeding cycle by between 0% and 40% for the vector species present at our four modeled sites.43–47 Bed nets can also lengthen the feeding cycle of anthrophilic mosquitoes, even when they shift substantially to biting non-human animals,44 but this shift has never been quantified. Therefore, in the interest of making a conservative estimate, we incorporate an impact of 15% extra mortality per feeding cycle and no impact on feeding cycle length.
Insecticides can also have the effect of repelling mosquitoes from dwellings46,48 and can discourage them from feeding upon humans.44,49 For example, at a site where the reported impact of impregnated bed nets on the survivorship of the vectors is very low, and indeed statistically insignificant, a shift to feeding on animals (a 30% drop in the human blood index, a measure of Q) and longer feeding cycles were demonstrated.44 Thus it seems that bed nets sometimes act by enhancing or enabling zooprophylaxis. A strong relationship between the human blood index of An. gambiae and the availability of cattle as alternative hosts has been demonstrated in The Gambia50 and at one of our Nigerian sites, Kaduna.51 In The Gambia, a cattle to human ratio of one or more reduced the human blood index of An. gambiae s.l. (mostly An. gambiae sensu stricto) to below 50%.50 Human blood indices of less than 50% have also been observed for both An. arabiensis and An. gambiae s.s. in a number of Kenyan communities which keep large numbers of cattle within their village compounds.52–55 Thus, although the presence of livestock has been known to increase malaria transmission intensity and clear guidelines are not available for most vectors,56 it appears that zooprophylaxis against malaria vectors is achievable in both sub-Saharan Africa and the southwest Pacific and may passively contribute to the effectiveness of bed nets. In order to incorporate the effects of livestock as decoys into our simulation of a bed-net program, we will include a moderate reduction to Q of 20%.
Modeling the impact of a malaria vaccine
Previous modeling studies have determined that regardless of the parasite stage targeted, the impact of any malaria vaccine on malaria transmission is equivalent to its level of efficacious coverage.57 Thus the impact of malaria vaccines on κcan be envisaged as a direct function of their efficacious coverage, which we set at 50%, a reasonable target for such vaccines and considerably better than that achieved by existing candidates.58
Modeling the impact of larval control
Reducing E, the vector emergence rate, represents a formidable task in most parts of Africa and Papua New Guinea. Nevertheless, methods do exist which can substantially reduce the availability of vector breeding sites in many situations.19 It has even been possible to eradicate, almost exclusively by larval control, the sub-Saharan African vector An. gambiae in both Brazil31 and Egypt32 after they were accidentally introduced there. Much of the larval habitat in both Butelgut41 and Kaduna34,51 are associated with rivers and streams, representing readily identifiable targets for larval control undertaken once a week or so. Although the topography of Kankiya was not well described, like Kaduna it is part of the dry savanna region of Nigeria. At Namawala, the striking peak of vector emergence observed annually is clearly associated with rice cultivation during the rainy season.36 Although methods do exist for vector control in such circumstances, they require rigorous and intensive implementation for complete success.31,32 The ability of all of the predominant vector species at these sites to breed in small, dispersed, temporary pools makes control even more difficult. Nevertheless, the complete eradication of An. gambiae s.l. from seemingly ideal habitats in flooding river valleys of northeast Brazil has proven that even this challenging vector is vulnerable to well-organized and determined larval control programs.31 Furthermore, a similar program in Egypt was effectively implemented at the peak of World War II, demonstrating that it can be achieved even with limited resources.32 For the purpose of a larval control campaign simulation which is conservative enough to be realistic but ambitious enough to be useful, we incorporate a two-fold (50%) reduction of emergence rates, to account for the level of larval habitat control that is possible with existing, cost-effective methods.19
RESULTS
The use of bed nets without controlling the infectiousness of the human parasite reservoir or larval habitats was predicted to reduce EIR by 7- to 13-fold at all four sites. Predicted reductions of the sporozoite rate (S) and the human biting rate (HBt) with bed nets were 3- to 5-fold and 2- to 3-fold, respectively. Correspondingly, the largest predicted impact of the integrated program on any of the three contributors to EIR is on L, the lifetime malaria transmission potential of individual mosquitoes (Figure 1B). However, the impact on L is amplified by two-fold reductions of E/Nh, the vector emergence rate relative to human population size (Figure 1C), and of κ, the infectiousness of the human reservoir (Figure 1A). This results in combined EIR reductions by the integrated program of between 30- and 50-fold (Figure 1D). Note, however, that the impact on HBt, the human biting rate, is not as striking, being approximately 5-fold (Figure 1E). This generalized control program was also predicted to have remarkably consistent effects on other relevant entomological parameters (Figure 2). The integrated control program reduced the lifetime biting capacity of individual vectors (bh) by only 2- to 3-fold but decreased their lifetime infectious biting capacity (β) by 15- to 27-fold. The integrated program reduced the sporozoite rates (S) and individual lifetime transmission potentials (L) of the vector populations by 6- to 11-fold and 8- to 13-fold, respectively.
Figure 1.
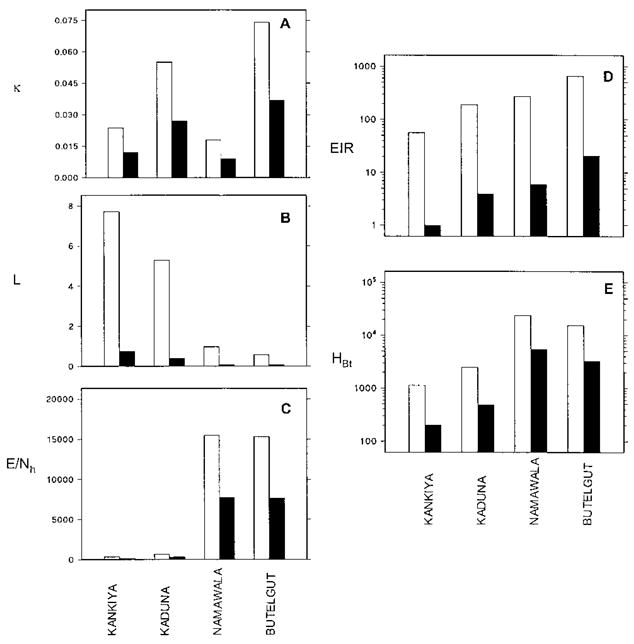
A: Simulated between-site variations in the size of the infectiousness of the human reservoir κ. B: The lifetime malaria transmission capacity of individual vectors (L); C: Mosquito emergence rate relative to the size of the human population E/Nh; D: The entomologic inoculation rate (EIR); and E: The human biting rate (HBt). Baseline conditions are represented by empty bars and those of an integrated transmission control program by solid bars. For further details of definitions and units see Killeen and others.1
Figure 2.
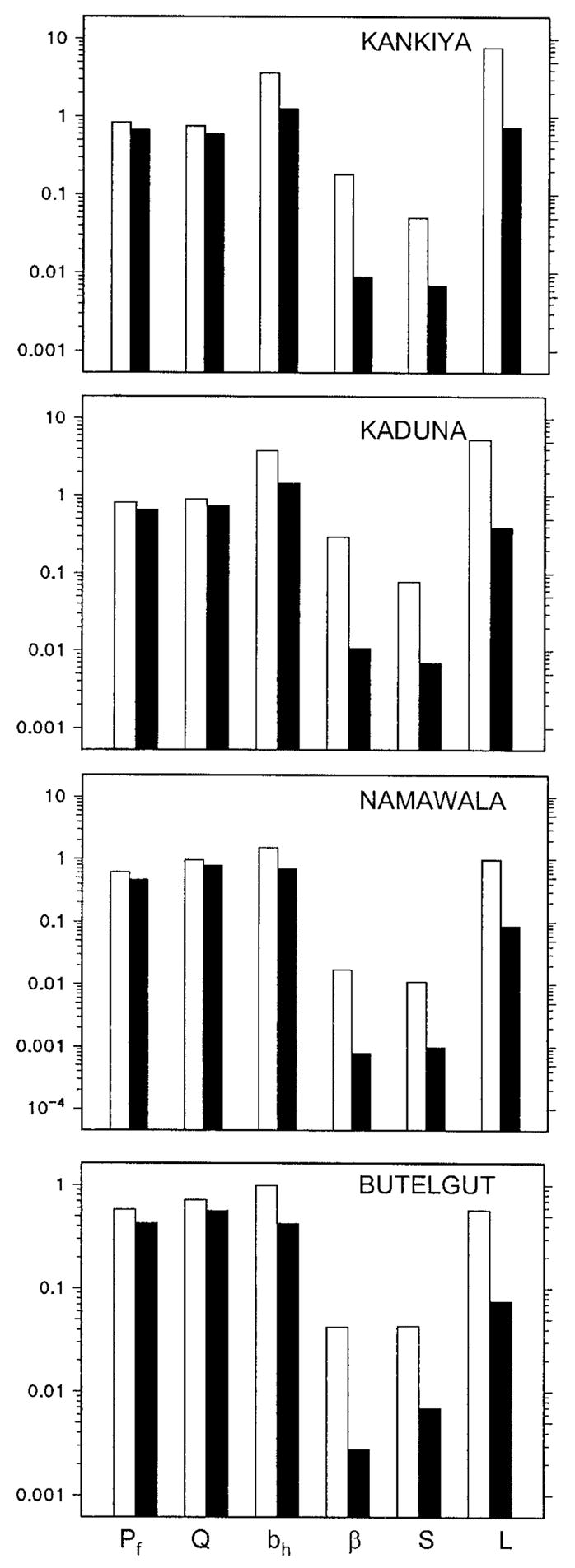
Predicted between-site variations in vector survival per feeding cycle (Pf), blood-feeding preferences (Q), number of human bites per lifetime (bh), number of infectious human bites per lifetime (β), sporozoite prevalence (S), and infectious bites per lifetime per bite on an infective human host (L) under baseline conditions (empty bars) and those of an integrated transmission control program (solid bars). See Killeen and others1 for further details of definitions and units.
DISCUSSION
This model yields outputs that are not only accurate under baseline conditions,1 but also predict the impact of domestic insecticide-based vector control remarkably well. The predictions of this model illustrate how a small decrease in vector populations can have a dramatic impact on EIR, and help explain the proven efficacy of bed nets and indoor spraying.12,14,20 The model demonstrates how integrated control using a few modestly effective tools can meaningfully reduce EIR but cannot eliminate malaria completely from areas of intense transmission.
The predicted impact of bed nets alone is strikingly consistent with observations made during insecticide-based control programs.42–47,59 Indeed, a DDT-spraying campaign at Kankiya reduced sporozoite rates and human biting rates by approximately 4- and 3-fold, respectively.33 These reductions are almost identical to the predictions of the model at this site, despite the a priori impact choices on the input parameters Pf and Q. The application of these predicted effects on EIR to the empirically established relationship between EIR and P. falciparum prevalence,2 allows us to estimate that 20–25% reductions in parasite prevalence can be expected. This compares well with observed impact in the field which varies from approximately 10% to 40% reductions in prevalence.59–62 Such large reductions of transmission intensity at the community level are consistent with the observed benefits of such programs, in terms of morbidity and mortality12,14,20 and their observed impact on unprotected people over considerable distances.63 Also, the modest reductions of bh, the total number of human bites a female mosquito will take, and hence the number of ovipositions she will complete during her lifetime (Figure 2) indicate that limited selective pressure will be exerted on the vector. This is consistent with the observation that pyrethroid resistance does not seem to emerge in vector populations exposed to impregnated bed nets in areas where these same insecticides are not used widely in agriculture.64
The ability of the model to explain the efficacy of bed nets further emphasizes the value of this model as a predictive tool for planning vector-control research and implementation.1 However, accurately verifying the impact of insecticide-based interventions on the EIR may be difficult in practice. For example, pyrethrum spray catches are likely to underestimate biting rates in dwellings which have insecticide-treated bed nets or walls, because repelled mosquitoes will exit after feeding.46,48 Conversely, standard human landing catches tend to overestimate the biting rate in communities with bed nets where residents are protected, but mosquito collectors are not.46 It is also important to bear in mind that the level of impact on Q, Pf, or f required to usefully reduce EIR is quite small and hence difficult to confirm statistically. This model demonstrates how the effects of bed nets may be explained by quite small reductions of these parameters and how the reduction of malaria burden can be achieved without decimating the vector population. Given that bed nets can exert their effects through any of the three parameters, accurate assessment of how transmission intensity is reduced in any trial probably requires detailed measurements of all of them.
The predictions of the model incorporating larval control and a malaria vaccine give an approximate estimate of the level of impact which can be achieved with affordable tools which are available now or likely to be available soon. Only one of the interventions included in the simulated transmission control program, an effective malaria vaccine, is unavailable at this time. Removing this intervention from the program still results in 15- to 25-fold reductions in EIR. Note also that these high reductions in EIR are accomplished with modest, 5-fold reductions in the overall human biting rate, HBt. This is important to bear in mind because HBt is the factor that communities often more readily perceive and are motivated by.62,65,66 Furthermore, in areas of very high transmission, a large impact on EIR may not be as readily appreciated as in areas of low transmission even though the impact, in terms of averted deaths, is equivalent.20 Because the overall disease burden in high transmission areas is much higher, reductions in mortality seem smaller when expressed and perceived as a proportion.20 Alleviation of mortality burden appears to be a consistent outcome, although the impact of transmission control in terms of protective efficacy is lower in areas with high baseline transmission intensity and with better medical facilities.12,14,20
The predicted impact of the combined control measures implies that an integrated approach is preferable for controlling intense malaria transmission and can achieve consistently large reductions of EIR, irrespective of baseline value. This is consistent with the fact that bed nets have roughly the same impact on absolute mortality over a wide range of transmission intensity.20 Clearly, integrated malaria transmission control should be implemented to the fullest extent possible in all endemic areas.2,12,14,20 Perhaps the most important question remaining with regard to bed nets and other transmission control measures is how to implement their widespread use rather than whether they are effective.12,20,67 We suggest that this model may be useful for deciding how to combine different tools when planning vector-control programs.1
Despite their dramatic predicted impact on EIR, the integrated control programs modeled here failed to reduce EIR to less than one at any of the four sites, even with the theoretical inclusion of a malaria vaccine. Endemic malaria with its concomitant disease burden is readily maintained by EIRs considerably below this level.2 Thus, although meaningful reductions in malaria burden can be achieved with existing technology, elimination from areas of intense transmission is currently beyond the grasp of many developing nations in Africa and the southwest Pacific. Techniques being explored at present include odor-baited traps, vector population replacement, vaccines, and biological control, but none of these interventions are likely to yield useful tools in the immediate future.68,69
The search for ways to combat malaria transmission should, perhaps, look as much to the past as to the future. In the interest of a conservative estimate, we incorporated a reduction of only 50% to vector emergence rates as being feasible at these sites. However, we suggest that far greater reductions can be achieved with existing tools, which have come to be regarded as old-fashioned. Although members of the An. gambiae complex, in particular, are notoriously difficult to control, complete elimination has been accomplished in both Brazil31 and Egypt32 in less than three years, even in areas with extensive seasonal flooding. Many of the habitats cleansed of An. gambiae in Brazil, appear to have been as ideal for An. gambiae proliferation as they were challenging to the implementation of control programs.31 The Egyptian campaign was executed with very limited resources during the peak of World War II, necessitating the use of sand rather than oil as a larvicide diluent.32 Despite these considerable obstacles, complete elimination was achieved in both instances by combining simple larvicides with effective, practical, and determined management of human resources.31,32 Although many of the principles of these vector-eradication campaigns are not practicable under the conditions of sustainable malaria transmission control programs, three key contributors are worth highlighting: 1) political support and freedom from bureaucratic obstacles, including full authority to enforce necessary measures, 2) clear chains of command and individualization of responsibilities, and 3) assignment of control and surveillance responsibilities to separate personnel.
Sustainable environmental tools for larval habitat source reduction and biological control are also well established,19 but remain grossly underused in most endemic areas. Such methods center on managing resources for food, water, and fuel. We suggest that such methods can meaningfully reduce malaria transmission intensity but that their broader application will require greater coordination between those concerned with malaria control and those concerned with agricultural, industrial, and economic development.
Acknowledgments
Valuable discussions and comments on the manuscript by Allan Saul, Susanne Straif-Bourgouise, and Barnett S. Cline were particularly helpful.
Financial support: The support of National Institutes of Health grants R01-AI-29000 (GFK, JCB), U19-AI-45511 (GFK, JCB), and F32-AI-1017 (FEM), and a Louisiana Educational Quality Scholarship Fund grant 1996-01-GF-23 (BDF) are gratefully acknowledged.
References
- 1.Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beier JC, Killeen GF, Githure J. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 3.Burkot TR, Graves PM, Paru R, Battistuta D, Barnes A, Saul A. Variations in malaria transmission rates are not related to anopheline survivorship per feeding cycle. Am J Trop Med Hyg. 1990;43:321–327. doi: 10.4269/ajtmh.1990.43.321. [DOI] [PubMed] [Google Scholar]
- 4.Stirnadel H, Beck HP, Alpers MP, Smith TA. Heritability and segregation analysis of immune responses to specific malaria antigens in Papua New Guinea. Genet Epidemiol. 1999;17:16–34. doi: 10.1002/(SICI)1098-2272(1999)17:1<16::AID-GEPI2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Rihet P, Traore Y, Abel L, Aucan C, Traore-Leroux T, Fumoux F. Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31-q33. Am J Hum Genet. 1998;63:498–505. doi: 10.1086/301967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rihet P, Abel L, Traore Y, Traore-Leroux T, Aucan C, Fumoux F. Human malaria: segregation analysis of blood infection levels in a suburban area and a rural area in Burkino Faso. Genet Epidemiol. 1998;15:435–450. doi: 10.1002/(SICI)1098-2272(1998)15:5<435::AID-GEPI1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Snow RW, Armstrong-Schellenberg JRM, Peshu N, Forster D, Newton CRJC, Winstanley PA, Mwangi I, Waruiru C, Warn PA, Newbold C, Marsh K. Periodicity and space-time clustering of severe childhood malaria on the coast of Kenya. Trans R Soc Trop Med Hyg. 1993;87:386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]
- 8.Jepson A, Sisay-Joof F, Banya W, Hassan-King M, Frodsham A, Bennett S, Hill AV, Whittle H. Genetic linkage of mild malaria to the major histocompatibility complex in Gambian children: study of affected sibling pairs. Br Med J. 1997;315:96–97. doi: 10.1136/bmj.315.7100.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert SC, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood BM, Whittle HC, Hill AV. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Snow R, Donnelly C, Newbold C. Aquired immunity and postnatal clinical protection in childhood cerebral malaria. Proc R Soc Lond B Biol Sci. 1999;266:33–38. doi: 10.1098/rspb.1999.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alles HK, Mendis KN, Carter R. Malaria mortality rates in South Asia and in Africa: implications for malaria control. Parasitol Today. 1998;14:369–375. doi: 10.1016/s0169-4758(98)01296-4. [DOI] [PubMed] [Google Scholar]
- 12.Lengeler C, Smith TA, Armstrong-Schellenberg J. Focus on the effect of bed nets on malaria morbidity and mortality. Parasitol Today. 1997;13:123–124. doi: 10.1016/s0169-4758(97)84870-3. [DOI] [PubMed] [Google Scholar]
- 13.Snow RW, Craig MH, Deichmann U, le Sueur D. A preliminary continental risk map for malaria mortality among African children. Parasitol Today. 1999;15:99–104. doi: 10.1016/s0169-4758(99)01395-2. [DOI] [PubMed] [Google Scholar]
- 14.Molineaux L. Malaria and mortality: some epidemiological considerations. Ann Trop Med Parasitol. 1997;91:811–825. doi: 10.1080/00034989760572. [DOI] [PubMed] [Google Scholar]
- 15.D’Alessandro U. Concerns on long-term efficacy of an insecticide-treated bednet programme on child mortality. Parasitol Today. 1997;13:124–125. doi: 10.1016/s0169-4758(97)84871-5. [DOI] [PubMed] [Google Scholar]
- 16.Snow RW, Omumbo JA, Lowe B, Molyneaux CS, Obiero JO, Palmer J, Weber MW, Pinder M, Nahlen B, Obonyo C, New-bold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 17.Snow RW, Marsh K. New insights into the epidemiology of malaria relevant to disease control. Br Med Bull. 1998;54:293–309. doi: 10.1093/oxfordjournals.bmb.a011689. [DOI] [PubMed] [Google Scholar]
- 18.Trape J-F, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 19.Rozendaal JA. Methods for Use by Individuals and Communities. Geneva: World Health Organization; 1997. Vector Control. [Google Scholar]
- 20.Lengeler C, Armstrong-Schellenberg J, D’Alessandro U, Binka F, Cattani J. Relative versus absolute risk of dying reduction after using insecticide-treated nets for malaria control in Africa. Trop Med Intl Health. 1998;3:286–290. doi: 10.1046/j.1365-3156.1998.00236.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogier C, Ly AB, Tall A, Cisse B, Trape JF. Plasmodium falciparum clinical malaria in Dielmo, a holoendemic area in Senegal: no influence of aquired immunity on initial symptomology and severity of malaria attacks. Am J Trop Med Hyg. 1999;60:410–420. doi: 10.4269/ajtmh.1999.60.410. [DOI] [PubMed] [Google Scholar]
- 22.Buckling AG, Taylor LH, Carlton JM, Read AF. Adaptive changes in Plasmodium transmission strategies following chloroquine chemotherapy. Proc R Soc Lond B Biol Sci. 1997;264:553–559. doi: 10.1098/rspb.1997.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogh B, Gamage-Mendis A, Butcher GA, Thompson R, Begtrup K, Mendis C, Enosse SM, Dgedge M, Barreto J, Eling W, Sinden RE. The differing impact of chloroquine and pyrimethamine/sulfadoxine upon the infectivity of malaria species to the mosquito vector. Am J Trop Med Hyg. 1998;58:176–182. doi: 10.4269/ajtmh.1998.58.176. [DOI] [PubMed] [Google Scholar]
- 24.Puta C, Manyando C. Enhanced gametocyte production in Fansidar-treated Plasmodium falciparum malaria patients: implications for malaria transmission control programmes. Trop Med Intl Health. 1997;2:227–229. doi: 10.1046/j.1365-3156.1997.d01-267.x. [DOI] [PubMed] [Google Scholar]
- 25.Lines JD, Wilkes TJ, Lyimo EO. Human malaria infectiousness measured by age-specific sporozoite rates in Anopheles gambiae in Tanzania. Parasitology. 1991;102:167–177. doi: 10.1017/s0031182000062454. [DOI] [PubMed] [Google Scholar]
- 26.Krogstad DJ. Malaria as a re-emerging disease. Epidemiol Rev. 1996;18:77–89. doi: 10.1093/oxfordjournals.epirev.a017918. [DOI] [PubMed] [Google Scholar]
- 27.Onori E, Grab B. Indicators for the forcasting of malaria epidemics. Bull Wld Hlth Org. 1980;58:91–98. [PMC free article] [PubMed] [Google Scholar]
- 28.Molyneux DH, Floyd K, Barnish G, Fevre EM. Transmission control and drug resistance in malaria: a crucial interaction. Parasitol Today. 1999;15:238–240. doi: 10.1016/s0169-4758(99)01453-2. [DOI] [PubMed] [Google Scholar]
- 29.Miller LH, Hoffman SL. Research toward vaccines against malaria. Nature Medicine. 1998;4(Supplement):520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 30.Kitron U, Spielman A. Suppression of transmission of malaria through source reduction: antianopheline measures applied in Israel, the United States, and Italy. Rev Infect Dis. 1989;11:391–406. doi: 10.1093/clinids/11.3.391. [DOI] [PubMed] [Google Scholar]
- 31.Soper FL, Wilson DB. Anopheles gambiae in Brazil. New York: The Rockefeller Foundation; 1943. [Google Scholar]
- 32.Shousha AT. Species-eradication. the eradication of Anopheles gambiae from Upper Egypt, 1942–1945. Bull Wld Hlth Org. 1948;1:309–353. [PMC free article] [PubMed] [Google Scholar]
- 33.Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae. Bull Wld Hlth Org. 1969;40:531–545. [PMC free article] [PubMed] [Google Scholar]
- 34.Service MW. The ecology of the mosquitoes of the Northern Guinea savannah of Nigeria. Bull Entomol Res. 1963;51:601–633. [Google Scholar]
- 35.Service MW. Some basic entomological factors concerned with the transmission and control of malaria in northern Nigeria. Trans R Soc Trop Med Hyg. 1965;59:291–296. doi: 10.1016/0035-9203(65)90010-6. [DOI] [PubMed] [Google Scholar]
- 36.Smith T, Charlwood JD, Kihonda J, Mwankusye S, Billingsley P, Meuwissen J, Lyimo E, Takken W, Teuscher T, Tanner M. Absence of seasonal variation in malaria parasitemia in an area of intense seasonal transmission. Acta Tropica. 1993;54:55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]
- 37.Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOL, Meuwissen JHET. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull Entomol Res. 1997;87:455–453. [Google Scholar]
- 38.Charlwood JD, Kihonda J, Sama S, Billingsley PF, Hadji H, Verhave JP, Lyimo E, Luttikhuizen PC, Smith T. The rise and fall of Anopheles arabiensis (Diptera: Culicidae) in a Tanzanian village. Bull Entomol Res. 1995;85:37–44. [Google Scholar]
- 39.Cattani JA, Moir JS, Gibson FD, Ginny M, Paino J, Davidson W, Alpers MP. Small-area variations in the epidemiology of malaria in Madang province. Papua New Guinea Med J. 1986;29:11–17. [PubMed] [Google Scholar]
- 40.Cattani JA, Tulloch JL, Vrbova H, Jolley D, Gibson FD, Moir JS, Heywood PF, Alpers MP, Stevenson A, Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- 41.Charlwood JD, Graves PM, Birley MH. Capture-recapture studies with mosquitoes of the group Anopheles punctulatus Donitz (Diptera: Culicidae) from Papua New Guinea. Bull Entomol Res. 1986;76:211–227. [Google Scholar]
- 42.Molineaux L, Gramiccia G. The Garki Project. Geneva: World Health Organisation; 1980. [Google Scholar]
- 43.Magesa SM, Wilkes TJ, Mnzava AEP, Njunwa KJ, Myamba J, Kivuyo MDP, Hill N, Lines JD, Curtis CF. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2 Effects on the malaria vector population. Acta Tropica. 1991;49:97–108. doi: 10.1016/0001-706x(91)90057-q. [DOI] [PubMed] [Google Scholar]
- 44.Charlwood JD, Graves PM. The effect of permethrin-impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Med Vet Entomol. 1987;1:319–327. doi: 10.1111/j.1365-2915.1987.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 45.Robert V, Carnevale P. Influence of deltamethrin treatment of bed nets on malaria transmission in the Kou Valley, Burkina Faso. Bull Wld Hlth Org. 1991;69:735–740. [PMC free article] [PubMed] [Google Scholar]
- 46.Quinones ML, Lines J, Thomson M, Jawara M, Greenwood BM. Permethrin-treated bedents do not have a “mass-killing effect” on village populations of Anopheles gambiae. Trans R Soc Trop Med Hyg. 1998;92:373–378. doi: 10.1016/s0035-9203(98)91053-7. [DOI] [PubMed] [Google Scholar]
- 47.Carnevale P, Robert V, Boudin C, Halna JM, Pazart L, Gazin P, Richard A, Mouchet J. La lutte contre le plaudisme par des moustiquaires impregnees de pyrethroides au Burkina Faso. Bull Soc Path Ex. 1988;81:832–846. [PubMed] [Google Scholar]
- 48.Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 49.Garrett-Jones C. The human blood index of malarial vectors in relationship to epidemiological assessment. Bull Wld Hlth Org. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- 50.Lindsay SW, Alonso PL, Armstrong-Schellenberg JRM, Hemingway J, Adiamah JH, Shenton FC, Jawa M, Greenwood BM. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 7. Impact of permethrin-impregnated bed nets on malaria vectors. Trans R Soc Trop Med Hyg. 1993;87(Supplement 2):45–51. doi: 10.1016/0035-9203(93)90175-p. [DOI] [PubMed] [Google Scholar]
- 51.White GB, Rosen P. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Diptera: Culicidae). II Ecology of species A and B in savanna around Kaduna, Nigeria, during transition from wet to dry season. Bull Entomol Res. 1973;62:613–625. [Google Scholar]
- 52.Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Tropica. 1994;58:307–316. doi: 10.1016/0001-706x(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 53.Highton RB, Bryan JH, Boreham PFL, Chandler JA. Studies on the sibling species Anopheles gambiae Giles and Anopheles arabiensis Patton (Diptera: Culicidae) in the Kisumu area, Kenya. Bull Entomol Res. 1979;69:43–53. [Google Scholar]
- 54.Ijumba JN, Mwangi RW, Beier JC. Malaria transmission potential of Anopheles mosquitoes in the Mwea-Tebere irrigation scheme, Kenya. Med Vet Entomol. 1990;4:425–432. doi: 10.1111/j.1365-2915.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 55.Service MW. Ecological notes on species A and B of the Anopheles gambiae complex in the Kisumu area of Kenya. Bull Entomol Res. 1970;60:105–108. [Google Scholar]
- 56.Mutero C, Mosha F, Oduluja A, Knols B, Bos R. Livestock management and malaria prevention in irrigation schemes. Parasitol Today. 1999;15:394–395. doi: 10.1016/s0169-4758(99)01522-7. [DOI] [PubMed] [Google Scholar]
- 57.Saul A. Minimal efficacy requirements for malaria vaccines to significantly lower transmission in epidemic or seasonal malaria. Acta Tropica. 1993;52:283–296. doi: 10.1016/0001-706x(93)90013-2. [DOI] [PubMed] [Google Scholar]
- 58.Graves PM. Comparison of the cost-effectiveness of vaccines and insecticide impregnation of mosquito nets for the prevention of malaria. Ann Trop Med Parasitol. 1998;92:399–410. doi: 10.1080/00034989859384. [DOI] [PubMed] [Google Scholar]
- 59.Modiano D, Petrarca V, Sirima BS, Nebie I, Luoni G, Esposito F, Coluzzi M. Baseline immunity of the population and impact of insecticide-treated curtains on malaria infection. Am J Trop Med Hyg. 1998;59:336–340. doi: 10.4269/ajtmh.1998.59.336. [DOI] [PubMed] [Google Scholar]
- 60.D’Alessandro U, Olaleye BO, McGuire W, Thomson MC, Langerock P, Bennet S, Greenwood BM. A comparison of the efficacy of insecticide-treated and untreated bednets in preventing malaria in Gambian children. Trans R Soc Trop Med Hyg. 1995;89:596–598. doi: 10.1016/0035-9203(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 61.Graves PM, Brabin BJ, Charlwood JD, Burkot TR, Cattani JA, Ginny M, Paino J, Gibson FD, Alpers MP. Reduction in incidence and prevalence of Plasmodium falciparum in under-5-year-old children by permethrin impregnation of mosquito nets. Bull Wld Hlth Org. 1987;65:869–877. [PMC free article] [PubMed] [Google Scholar]
- 62.Thomson MC, D’Alessandro U, Bennet S, Connor SJ, Langerock P, Jawara M, Todd J, Greenwood BM. Malaria prevalence is inversely related to vector density in The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1994;88:638–643. doi: 10.1016/0035-9203(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 63.Binka FN, Indome F, Smith T. Impact of spatial distribution of permethrin-impregnated bed nets on child mortality in rural Northern Ghana. Am J Trop Med Hyg. 1998;59:80–85. doi: 10.4269/ajtmh.1998.59.80. [DOI] [PubMed] [Google Scholar]
- 64.Vulule JM, Beach RF, Atieli FK, Mount DL, Roberts JM, Mwangi RW. Long-term use of permethrin-impregnated nets does not increase Anopheles gambiae permethrin tolerance. Med Vet Entomol. 1996;10:71–79. doi: 10.1111/j.1365-2915.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 65.Lines J. Mosquito nets and insecticides for net treatment: a discussion of existing and potential distribution systems in Africa. Trop Med Int Health. 1996;1:616–632. doi: 10.1111/j.1365-3156.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 66.Gyapong M, Gyapong JO, Amankwa J, Asedem J, Sory E. Introducing insecticide impregnated bednets in an area of low bednet usage: an exploratory study in north-east Ghana. Trop Med Int Health. 1996;1:328–333. doi: 10.1046/j.1365-3156.1996.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 67.Lengeler C, Snow RW. From efficacy to effectiveness: insecticide treated bednets in Africa. Bull Wld Hlth Org. 1996;73:325–332. [PMC free article] [PubMed] [Google Scholar]
- 68.Curtis CF, Townson H. Malaria: existing methods of vector control and molecular entomology. Br Med Bull. 1998;54:311–325. doi: 10.1093/oxfordjournals.bmb.a011690. [DOI] [PubMed] [Google Scholar]
- 69.Takken W, Knols BG. Odor-mediated behavior of Afrotropical mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]


