Abstract
Phytophthora ramorum and Phytophthora sojae are destructive plant pathogens. P. sojae has a narrow host range, whereas P. ramorum has a wide host range. A global proteomics comparison of the vegetative (mycelium) and infective (germinating cyst) life stages of P. sojae and P. ramorum was conducted to identify candidate proteins involved in host range, early infection, and vegetative growth. Sixty-two candidates for early infection, 26 candidates for vegetative growth, and numerous proteins that may be involved in defining host specificity were identified. In addition, common life stage proteomic trends between the organisms were observed. In mycelia, proteins involved in transport and metabolism of amino acids, carbohydrates, and other small molecules were up-regulated. In the germinating cysts, up-regulated proteins associated with lipid transport and metabolism, cytoskeleton, and protein synthesis were observed. It appears that the germinating cyst catabolizes lipid reserves through the β-oxidation pathway to drive the extensive protein synthesis necessary to produce the germ tube and initiate infection. Once inside the host, the pathogen switches to vegetative growth in which energy is derived from glycolysis and utilized for synthesis of amino acids and other molecules that assist survival in the plant tissue.
Organisms of the genus Phytophthora are destructive plant pathogens capable of infecting many agriculturally and ornamentally important crops (1). To date, there are over 80 recognized species of Phytophthora. Although some Phytophthora species are able to infect a broad range of host plants, others are limited to a single host. Phytophthora ramorum and Phytophthora sojae are examples of each of these groups, respectively. The recently characterized P. ramorum is the causal agent of sudden oak death disease (2, 3). In addition to its destructive effect on live oak trees, as currently unfolding in forests in California and Oregon, P. ramorum is capable of infecting a wide range of trees and shrubs, such as bay laurel and viburnum (4). On the other hand, P. sojae, the causal agent of soybean root and stem rot, has a very narrow host range. Races of P. sojae are cultivar-specific with certain P. sojae isolates only infecting certain varieties of soybean (1).
The genomes of P. sojae and P. ramorum have been sequenced recently (5). The genome of P. sojae is 95 Mbp and predicted to encode 19,027 genes, whereas the genome of P. ramorum is 65 Mbp and predicted to encode 15,743 genes. When compared with one another, the two organisms have a high degree of orthology and synteny between their genomes (5, 6). Thus, despite the similarity between the two organisms, there are obviously specific differences that define the organisms in terms of their unique properties. One aim of this study was to identify candidate proteins that might be involved in host range capacity. This was achieved by comparing the expressed proteomes of P. sojae and P. ramorum and identifying differences between them.
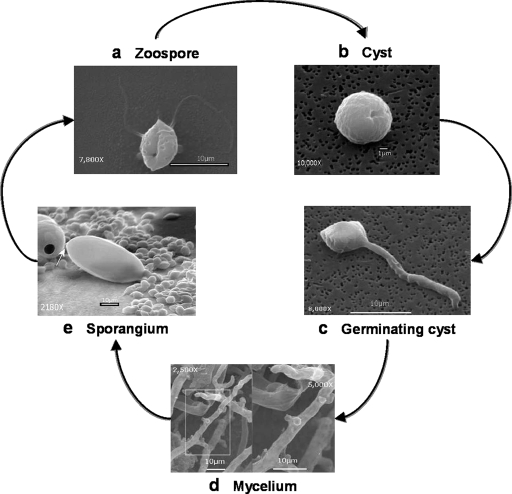
Phytophthora species are capable of reproducing sexually and asexually. During the sexual life cycle oospores, the sexual spores, are produced. Oospores have thick cell walls and can survive in the soil for years, thus allowing reinfection of their host plant in subsequent growing seasons. However, because the oospores require a dormancy period of several weeks before germination, it is the asexual life cycle that is responsible for rapid propagation and spread of the disease in the field or forest. During the asexual life cycle, the organism is able to differentiate into different life stages including mycelium, sporangium, zoospore, cyst, and germinating cyst (Fig. 1).
Fig. 1.
The asexual life cycle of Phytophthora. Scanning electron micrographs of asexual life stages of P. ramorum are shown. Motile biflagellated zoospores (a) swim chemotactically toward its plant host. Upon contact with the plant (or shaking in the laboratory) the zoospore sheds its flagella and encysts (b). The cyst germinates (c), and the germ tube penetrates into the plant tissue where it starts growing vegetatively through the plant tissue as mycelium (d). The mycelium can grow out of the plant tissue where, under appropriate stimulation, its terminal ends can differentiate into sporangia (e), which are structures containing multiple nuclei that can differentiate into zoospores. Zoospores can then be released from the sporangia and repeat the cycle. Micrographs were supplied courtesy of Dr. Edwin R. Florance, Lewis & Clark College, Portland, OR.
Infection of a plant can be initiated when a zoospore, a motile kidney-shaped biflagellated cell, interacts with a compatible plant host. Upon contact with the plant host the zoospore sheds its flagella, encysts, and adheres. Shortly after encystment the cyst germinates by producing a germ tube that penetrates the host tissue directly or through wounds or natural openings. Once inside the plant, the pathogen grows and ramifies through the plant tissue as mycelium, the vegetative growth life stage of Phytophthora. In a compatible interaction the initial growth of the mycelium in the plant tissue is biotrophic where the pathogen evades the plant defense responses. Later growth is switched to necrotrophic, and the plant tissue is destroyed. A second aim of this study was to identify candidate proteins involved in initiation of infection. This was achieved by comparing the proteomes of the germinating cyst and the mycelium life stages for both organisms.
Proteomics investigation of the mycelium and germinating cyst life stages from P. sojae and P. ramorum was carried out using multidimensional protein identification technology (MudPIT)1 (7). Because this technique provides a relatively unbiased way to rapidly characterize a more significant portion of the proteome than two-dimensional gel electrophoresis, it has been used extensively on many organisms (i.e. Refs. 7–10). Several groups compared the identified proteomes of an organism grown under different treatments (comparative proteomics) to elucidate biological responses to these treatments (11–13). Comparative proteomics was also applied to different life stages of eukaryotic organisms to gain insight to their life cycle (14, 15). Here we present an approach incorporating comparative proteomics with orthology to investigate cellular processes in closely related organisms. The rationale is that orthologous proteins that are up-regulated in a certain life stage in both organisms may be specifically involved in that life stage, whereas proteins with no similarly expressed orthologs, or with no orthologs at all, may contribute specific functions to that organism. In our case, orthologous proteins that were up-regulated in germinating cyst both in P. sojae and P. ramorum were identified as candidates involved in cyst germination and therefore may be involved in early infection. Orthologous protein pairs that were up-regulated in mycelium were identified as candidates important for vegetative growth. Proteins expressed uniquely in P. ramorum were identified as candidates allowing its broad host range capacity, whereas uniquely expressed P. sojae proteins were identified as candidates for defining its narrow host range. In addition, the methodological approach presented here includes use of orthology and a variety of novel bioinformatics strategies to analyze the data and represents a step forward in the analysis of proteomics data from closely related species.
EXPERIMENTAL PROCEDURES
Phytophthora Cell Culture, Life Stage Isolation, Lysate Preparation, and LC/LC-MS/MS Analysis—
Cell tissue was prepared as described previously (16). Briefly mycelia were generated by growing P. sojae strain P6497 (University of California, Riverside) and P. ramorum strain LT191 (University of California, Riverside) in clarified antibiotic-amended V8 juice broth. Asexual sporangia and zoospores were generated by growing P. sojae and P. ramorum on antibiotic-amended V8 agar plates according to standard protocols (1). Zoospores were stimulated to encyst by vigorous shaking for 1–2 min and then waiting ∼1 h before harvesting the germinating cysts. The mycelium and germinating cyst were freeze-dried and ground using glass beads.
Lysates were fractionated to membrane and soluble fractions by centrifugation, and the soluble fractions were digested with trypsin after denaturation, reduction, and alkylation as described previously (16). Filtered and acidified samples were then loaded off line on a biphasic 150-μm-inner diameter fused silica column containing 3–3.5 cm of C18 reverse phase material followed by 3.5–4 cm of strong cation exchange phase. Loaded columns were placed directly upstream of a 100-μm-inner diameter front column packed with 15 cm of C18 reverse phase material. Samples were analyzed by 11-step MudPIT (LC/LC-MS/MS) on a linear ion trap mass spectrometer (LTQ, ThermoElectron, San Jose, CA) as described previously (16). Flow rate at the tip was ∼200–300 nl/min.
Informatics—
MS/MS spectra from RAW files of MudPIT experiments were extracted into MS2 files using Raw2MS2.exe (17, 18). Spectra were then assigned charge using MS2ZAssign (18) and searched against the predicted protein databases using the DBDigger algorithm (19) in fully tryptic mode with the Multinomial Algorithm for Spectral Profile-based Intensity Comparison (MASPIC) scorer (18). No limit on missed cleavages was specified. The P. sojae protein database used was Psojae_proteins_VMD_V-1.0, release date July, 29, 2004, and included 19,027 P. sojae proteins as well as 52 common contaminants. The P. ramorum protein database used was Pramorum_proteins_VMD_V-1.0, release date July 29, 2004, and included 15,743 P. ramorum proteins as well as 52 common contaminates. Search results were filtered with the DTASelect algorithm (20) using filter values allowing a 5% false discovery rate (supplemental Table S1) based on a concatenated reversed protein database search as described previously (16). Briefly spectra from one representative MudPIT experiment for each organism were searched against a protein database containing the respective organism's protein sequences, the contaminants, and the reversed sequences of both proteins and contaminants (essentially representing random entries with the same amino acid composition and length). Filter values allowing one hit to the reverse database for every 19 to the forward database (5% false discovery rate) were determined using the SQTRevPuller algorithm (21) and applied thereafter to all search results for the same organism. For all searches, a fixed modification of +57 Da on cysteine residues was included as cysteines were considered to be fully carboxamidomethylated. No variable modifications were included. Protein identification required two peptides per protein for identification. Mass tolerance for precursor ions was 3 Da, and mass tolerance for fragment ions was 0.5 Da. Comparison between protein lists was carried out using Microsoft Access and Perl scripts. To make comparisons between life stages, the union of the duplicate MudPIT results of the germinating cyst was compared with the union of the duplicate MudPIT results of the mycelium from the same organism.
Spectral count was considered as the total number of MS/MS spectra corresponding to peptides from a given protein. Normalization of spectral counts was done using normalized spectral abundance factors (NSAFs) as described previously (22). Proteins groups that shared all of their peptides were assigned an NSAF value equal to the NSAF value for the group divided by the number of proteins in the group. A protein was determined to be up-regulated in one life stage versus another if (i) it was identified in both MudPIT duplicates of the up-regulated life stage and (ii) the sum of the normalized spectral counts from both duplicates was at least 5 times higher than the sum of normalized spectral counts in the other life stage duplicates. For statistical significance, a G-test was performed such that for each protein the sums of normalized spectral counts in each life stage were tested against the null hypothesis that they are not different from an expected 1:1 ratio with a two-tailed p value <0.05.
Gene Ontology (GO) annotations as well as Eukaryotic Orthologous Groups (KOG) annotations were downloaded from the United States Department of Energy Joint Genome Institute Website into a Microsoft Access relational database and associated with their corresponding protein IDs. Signal peptide assignment was done using a local copy of SignalP (Center for Biological Sequence Analysis, Technical University of Denmark).
Orthology was determined by reciprocal BLASTp of the P. sojae and P. ramorum protein databases. A pair of proteins was assigned orthology if both proteins were the best hit on their partner's BLAST results and had an E value <10−50 (supplemental Table S2). Differential expression between orthologous proteins was determined as described above for a protein in different life stages.
To make comparisons between the two organisms, the union of the duplicate MudPIT results of each life stage from P. ramorum was compared, based on orthology, with the union of duplicate MudPIT results from the same life stage in P. sojae. For identification of host range-specific candidates, the raw lists of organism-specific proteins (supplemental Tables S3 and S4) were manually examined. Steps that were taken to identify host range-specific candidate from the raw lists included (a) high spectral count for confident protein identification and abundance, (b) observation of KOG and GO annotation, (c) BLASTp against the other organism protein database for identification of homologs with similar expression, (d) BLASTp against the non-redundant protein database for identification of homologs in other species, (e) ClustalW alignment with homologous proteins to confirm homology, (f) Pfam analysis for domain identification, and (g) literature search. All raw data and the supporting analysis are available upon request.
RESULTS AND DISCUSSION
Comparison of the Global Proteomes of Germinating Cyst and Mycelium Life Stages—
Infection of a plant host by Phytophthora can be initiated when a Phytophthora zoospore adheres and encysts on the plant tissue, produces a germ tube, gains access to the plant host, and starts growing vegetatively throughout the plant tissue as mycelium. We investigated the proteomic differences between the germinating cyst and mycelium life stages from P. sojae and P. ramorum by MudPIT to identify candidate proteins that might be involved in early infection. Duplicate MudPIT experiments were carried out on each of the life stages from P. ramorum and P. sojae. Altogether 3897 P. ramorum and 2970 P. sojae proteins were identified. In the germinating cyst 2065 and 2089 P. ramorum proteins were identified in the first and second replicates (supplemental Tables S5 and S6), respectively, with 1293 of them found in both replicates (63% reproducibility). In the P. ramorum mycelium 1966 and 1962 proteins were identified in the first and second replicates (supplemental Tables S5 and S6), respectively, with 1339 of them found in both replicates (68% reproducibility). For P. sojae 1940 and 1779 germinating cyst proteins were identified in the first and second replicates (supplemental Tables S5 and S7), respectively, with 1248 of them found in both replicates (70% reproducibility). In the P. sojae mycelium 1313 and 1216 proteins were identified in the first and second replicates (supplemental Tables S5 and S7), respectively, with 825 of them found in both replicates (68% reproducibility). It should be noted that the major factor in reducing reproducibility comes from identification of low abundance proteins from which two peptides happened to be sampled in one replicate but not the other. For abundant proteins the reproducibility rate was much higher than stated above.
One group of proteins that was identified in both life stages of both organisms included superoxide dismutases, catalases, and peroxidases. These enzymes break down reactive oxygen species (23–26) that are produced as by-products of aerobic respiration as well as by plant defense mechanisms in response to pathogens (27–29). It has been shown that pathogenesis of certain bacteria and fungi can be correlated to their reactive oxygen species-inactivating enzymes (30–32). Thus, constituent expression of these proteins in Phytophthora may contribute to their success as plant pathogens.
On average, 14% of all identified proteins in each organism had a predicted signal peptide and are thus likely secreted or membrane proteins. This number of secreted or membrane proteins is high, especially considering that only an estimated 8% of all predicted genes in the genome are predicted to be secreted (5, 6) and that the protein preparations were not designed specifically to contain such proteins (only crude soluble fractions of total lysates were analyzed). Among others, these signal peptide-containing proteins included peptidases and other hydrolases, chaperones, transporters, and structural molecules. Among the secreted proteins, 42 P. sojae and 46 P. ramorum proteins contained the RXLR motif within the first 30–60 residues of the N terminus, possibly classifying them as members of the RXLR family, a group of effector proteins that is thought to be targeted to the host cell cytosol where they manipulate host defense responses (33–35).
Relative Protein Expression—
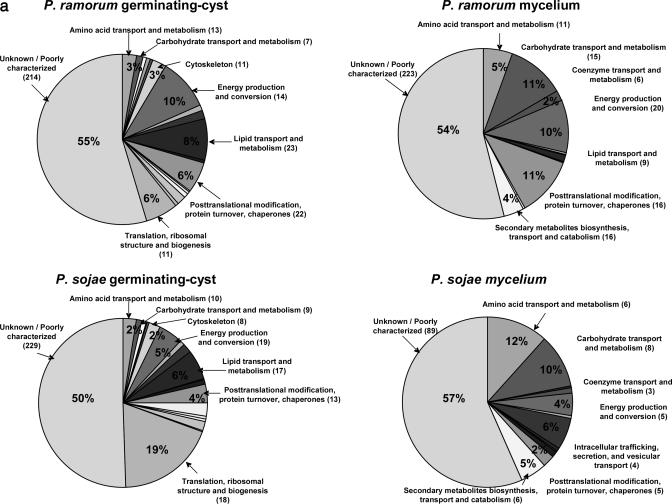
For relative protein expression analysis, protein abundance was determined based on normalized spectral counts (see “Experimental Procedures”). Spectral count has been shown to provide a reliable parameter for estimation of relative protein abundance in proteomes up to at least 3 orders of magnitude (36). Normalization of spectral count according to published protocols (22) was done to meaningfully compare protein abundances across different experiments. Within the same experiment normalization minimizes the bias of higher spectral count number toward longer proteins, and between different experiments it minimizes differences that may arise due to variation in experimental procedures (initial protein concentration in each sample, protein digestion efficiency, instrument performance, etc.). Each identified protein was associated with its GO and KOG annotations, and proteins were clustered into functional groups based on their KOG annotation. The proportion of each functional category of the total was calculated based on the sum of normalized spectral counts of all proteins in each category. A breakdown of the identified proteomes of the mycelium and germinating cyst life stages of P. ramorum and P. sojae is shown in Fig. 2. Very few functional categories changed significantly, and the proportion of most categories remained fairly constant between germinating cyst and mycelium (Fig. 2). These results indicate that although some distinct processes are taking place in each life stage other common functions are being carried out in both.
Fig. 2.
Distribution of protein functional categories in the global proteomes of the germinating cyst and mycelium of P. ramorum and P. sojae. Each identified protein was associated with its KOG annotation, and protein categories were clustered based on their KOG functional annotation. The proportion of each protein of the total was calculated based on NSAFs as described previously (22). a, each pie chart represents the union of duplicate MudPIT experiments. The numbers in parentheses represent the number of proteins in the respective functional category. b and c, for comparison purposes, all functional categories with >5% of the total are presented as column charts.
To focus on cellular processes that are unique to each of the life stages, subset proteomes were compiled that contained only those proteins that were differentially expressed between germinating cyst and mycelium. A protein was considered to be up-regulated in a specific life stage if its abundance, based on normalized spectral counts, was at least 5-fold higher than in the other life stage (see “Informatics” under “Experimental Procedures”). 686 proteins were determined to be differentially expressed between germinating cyst and mycelium for P. ramorum (supplemental Table S8 and Fig. 3), and 513 proteins were determined to be differentially expressed between germinating cyst and mycelium for P. sojae (supplemental Table S9 and Fig. 3). A G-test on normalized spectral counts between the life stages returned a statistical significant difference for all of these differentially expressed targets (with a p value <0.05) (data not shown). When examining only the differentially expressed proteins, the proteomic differences between germinating cyst and mycelium become more apparent (Fig. 4). Thus, ignoring the common background (i.e. housekeeping proteins with equivalent abundance in both life stages) aids in emphasizing the differences between the two. Interestingly the proportion of unknown proteins increases dramatically in the differentially expressed protein sets as compared with the global proteome analysis (compare Fig. 2 with Fig. 4), suggesting that many life stage-specific proteins have yet to be characterized in these or other related organisms.
Fig. 4.
Distribution of protein functional categories in the differentially expressed proteomes of the germinating cyst and mycelium of P. ramorum and P. sojae. Each identified protein was associated with its KOG annotation, and protein categories were clustered based on their KOG functional annotation. The proportion of each protein of the total was calculated based on NSAFs as described previously (22). Only proteins that were determined to be differentially expressed (see “Experimental Procedures”) were included in this analysis. a, each pie chart represents the union of duplicate MudPIT experiments. The numbers in parentheses represent the number of proteins in the respective functional category. b and c, for comparison purposes, all functional categories with >5% of the total (with the exception of the “unknown” category) are presented as column charts.
Germinating Cyst Proteome—
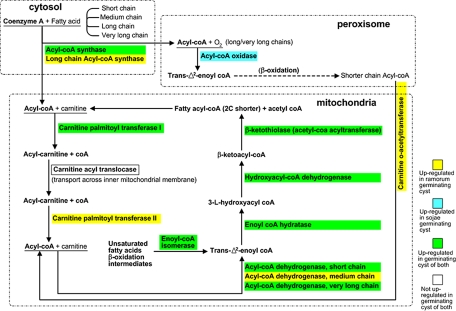
In the germinating cyst life stage from both P. sojae and P. ramorum, three KOG categories showed a substantial increase in expression as compared with mycelium: translation, ribosomal structure, and biogenesis (protein synthesis); cytoskeleton; and lipid transport and metabolism (Fig. 4). Up-regulated proteins in the lipid transport and metabolism category included an array of proteins spanning the entire β-oxidation pathway, which is the process of fatty acid catabolism (37) (Fig. 5). These include acyl-CoA synthases (activates fatty acids), carnitine O-acyltransferase (transports peroxisomal activated fatty acids into the mitochondria), acyl-CoA dehydrogenase (catalyzes fatty acid oxidation), and hydroxyacyl-CoA dehydrogenase (subsequent oxidation of fatty acids) among others. Based on this, it appears that the source of energy production prior to penetration and harvesting of host resources is via lipid catabolism. A previous study in Phytophthora infestans found one component of this pathway, acyl-CoA synthetase, to be up-regulated in germinating cyst (38), leading the authors to hypothesize lipid metabolism as one of several possible energy production resources. Considering this, our results suggest that germinating cyst β-oxidation may be applicable across the entire genus. Although it occurs in peroxisomes and mitochondria of mammals and some algae, β-oxidation occurs exclusively in peroxisomes of plants and fungi (37, 39). Both peroxisome- and mitochondrion-specific proteins were identified, suggesting β-oxidation in both organelles. Thus, β-oxidation of fatty acids in both organelles suggests a closer evolutionary connection of Phytophthora to animals than to plants or fungi.
Fig. 5.
Lipid catabolism via the β-oxidation pathway. Multiple enzymes involved in the catabolism of fatty acids were found to be up-regulated in the germinating cyst of P. sojae and P. ramorum. For simplicity, the cofactors, reducing agents, water molecules, ATP molecules, etc. were excluded from the diagram, leaving only the proteins and substrates. The diagram is based on Kunau et al. (37).
An increase in the translation, ribosomal structure, and biogenesis category and cytoskeleton category is consistent with the process of cyst germination. During this process, the cyst produces a long germ tube that, during infection, penetrates host plant tissue. Presumably production of the germ tube requires large amounts of cytoskeleton proteins as well as other proteins, and thus a concomitant increase in the mentioned categories is expected. Supporting this notion is the fact that in both organisms several proteins associated with microtubule-based movement were up-regulated in the germinating cyst life stage but were absent or down-regulated in mycelium. These proteins may be involved in shuttling specific proteins destined for interaction with the host to the extremities of the germ tube. An increase in the translation, ribosomal structure, and biogenesis category may also represent ramping of protein production in the transition from a cyst to vegetative growth as mycelium. These results are consistent with previous studies (38, 40–42) that suggested up-regulation of de novo RNA and protein synthesis in the germinating cyst of other Phytophthora species.
Notably lipid metabolism via the peroxisomal β-oxidation pathway has been shown to be essential for Arabidopsis seedling growth (43–46). Components of the β-oxidation pathway have also been shown to be involved in the control of seed germination in Arabidopsis (47, 48) and have been shown to play a critical role in appressorium (the preinfective structure produced by the germinating cyst) function of the fungus Magnaporthe grisea (49). Cytoskeleton proteins such as actin and tubulin have also been shown to be important in germination of Arabidopsis seed (50) and rice pollen (49) as well as in spore germination of filamentous fungi such as Aspergillus nidulans (51). Finally increased protein synthesis has also been shown to occur in plant seed, plant pollen, and fungal spore germination (49, 52, 53). All of these point to potentially conserved mechanisms for germination for plants, fungi, and oomycetes.
In Arabidopsis it has been shown that protein synthesis during seed germination is dependent on mRNA that has been presynthesized during seed maturation (53). Although it was abolished in the presence of a translational inhibitor, seed germination was able to proceed in the absence of transcription. The fact that a large increase was observed for the translation, ribosomal structure, and biogenesis category in the germinating cyst (Fig. 4) but no increase was observed for the transcription category (remained less than 1%) may suggest a similar mechanism in which protein synthesis in the germinating cyst is dependent on mRNA presynthesized earlier in the life cycle, perhaps during zoospore encystment.
63 orthologous protein pairs were up-regulated in the germinating cyst of both organisms (Fig. 3 and supplemental Table S10). Thus, they were identified as candidates for involvement in early infection. A group of four such orthologous protein pairs was highly up-regulated in the germinating cyst of both organisms but completely undetected in the mycelium samples (one example in Table I). These proteins were annotated as structural molecules, and although they vary in sequence, they all share multiple “laminin-type epidermal growth factor (EGF)-like” domains. The EGF-like domain is a sequence of 30–40 amino acids found in EGF as well as in many other proteins from various organisms (54). This domain is typically found in the extracellular domain of membrane-bound proteins or in secreted proteins (54, 55) that typically mediate cell adhesion, growth migration, and differentiation (56–59). All Phytophthora proteins in this group are predicted to contain a signal sequence, indicating that these proteins may be acting at the interface between the host and the pathogen. These proteins may play a part in adhesion of the cyst to the plant host and facilitate both germination of the cyst on the plant tissue and penetration of the germ tube into the host tissue. BLAST searches of these Phytophthora proteins showed high sequence similarity to the products of three sexually induced genes (SIG1, SIG2, and SIG3) in the diatom Thalassiosira weissflogii. The SIG proteins also show a striking similarity to other extracellular proteins involved in cell-cell interactions (60), supporting the hypothesis that the corresponding P. sojae and P. ramorum proteins are involved in interaction with their plant hosts.
Fig. 3.
Identification of candidate proteins for involvement in early infection or vegetative growth. For each organism, the total identified proteomes of the germinating cyst and mycelium were compared (top and bottom overlapping circles). The non-overlapping regions represent the differentially expressed proteins in each life stage, whereas the overlapping regions represent the rest of the identified proteins. Orthologous proteins that were up-regulated in the germinating cyst life stage of both organisms (overlapping region, middle left) were identified as candidates for early infection. Orthologous proteins that were up-regulated in the mycelium life stage of both organisms (overlapping region, middle right) were identified as candidates for vegetative growth.
Table I.
Examples of candidate proteins for life stage specificity or host range
| Candidatea number | Organism | Protein ID | Up-regulation life stage | -Fold increase | Spectral count cyst/mycb | Function | Possible role |
|---|---|---|---|---|---|---|---|
| 1 | P. ramorum | 74450 | Germinating cyst | >5 | 19/0 | Structural molecule, laminin-type EGF-like domain | Early infection |
| 1 | P. sojae | 140046 | Germinating cyst | >5 | 91/0 | Structural molecule, laminin-type EGF-like domain | Early infection |
| 2 | P. ramorum | 83420 | Germinating cyst | >5 | 248/0 | Unknown, ricin domain | Early infection |
| 2 | P. sojae | 142672 | Germinating cyst | 9 | 159/13 | Unknown, ricin domain | Early infection |
| 3 | P. ramorum | 87864 | Germinating cyst | 6 | 44/10 | Unknown, α-mannosidase similarity | Early infection |
| 3 | P. sojae | 145478 | Germinating cyst | 17 | 75/4 | Unknown, α-mannosidase similarity | Early infection |
| 4 | P. ramorum | 80246 | Germinating cyst | 8 | 131/27 | α-Mannosidase | Early infection |
| 4 | P. sojae | 131780 | Germinating cyst | 5 | 184/28 | α-Mannosidase | Early infection |
| 5 | P. ramorum | 75997 | Mycelium | 14 | 6/107 | Coproporphyrinogen-III oxidase | Vegetative growth |
| 5 | P. sojae | 132631 | Mycelium | 5 | 4/16 | Coproporphyrinogen-III oxidase | Vegetative growth |
| 6 | P. ramorum | 85749 | Mycelium | 20 | 4/123 | Annexin | Vegetative growth |
| 6 | P. sojae | 144346 | Mycelium | >5 | 0/91 | Annexin | Vegetative growth |
| 7 | P. ramorum | 87858 | Mycelium | >5 | 0/179 | Annexin | Vegetative growth |
| 7 | P. sojae | 145567 | Mycelium | >5 | 0/7 | Annexin | Vegetative growth |
| 8 | P. ramorum | 77742 | Mycelium | 8 | 6/77 | Phospholipase D | Vegetative growth |
| 8 | P. sojae | 127024 | Mycelium | 13 | 7/64 | Phospholipase D | Vegetative growth |
| 9 | P. sojae | 127028 | Germinating cyst | >5 | 28/0 | Glycosyl hydrolase family 81 | Soybean infection |
| 10 | P. sojae | 126853 | Germinating cyst | >5 | 13/0 | Similar to CRN3 from P. infestans | Soybean infection |
| 11 | P. sojae | 145528 | Germinating cyst | >5 | 17/0 | Similar to CRN5 from P. infestans | Soybean infection |
| 12 | P. sojae | 140694 | Mycelium | 11 | 7/57 | CBEL, cell surface glycoprotein | Soybean infection |
| 13 | P. ramorum | 78735 | Mycelium | >5 | 0/51 | Nucleoside-diphosphate-sugar epimerase | Broad host range |
| 13 | P. sojae | 133984 | 0/0 | Nucleoside-diphosphate-sugar epimerase | |||
| 14 | P. ramorum | 76706 | Mycelium | >5 | 0/30 | Sugar transporter of the major facilitator superfamily | Broad host range |
Proteins with the same candidate number are orthologs. Individual candidates have no orthologs.
Mycelium.
Another pair of similarly regulated orthologs includes P. ramorum protein ID 83420 and P. sojae protein ID 142672. Both proteins were highly up-regulated in germinating cyst (Table I). Although the function of these proteins is unknown, they contain multiple ricin-B lectin domains. Ricin is a lectin found in castor beans and is toxic to people, animals, and insects. The ricin-B lectin domain is found in many carbohydrate recognition proteins including plant and bacterial AB-toxins, glycosidases, or proteases (61–63). Up-regulation in the germinating cyst life stage may be directed at interaction with host carbohydrate moieties such as sugars or glycoproteins as a part of the chemical warfare that occurs between the pathogen and the host.
A final example of proteins potentially involved in early infection includes two orthologous pairs annotated as lysosomal α-mannosidases. These proteins were all up-regulated in germinating cyst (Table I). Lysosomal α-mannosidase is an enzyme that mediates the catabolism of N-linked carbohydrates released during the glycoprotein degradation process (64). An increase in expression of these proteins in the germinating cyst may indicate increased internal glycoprotein turnover in the transition from cyst to mycelium or perhaps even degradation of secreted host glycoprotein taken up by the pathogen.
Mycelium Proteome—
When compared with germinating cyst, the portion of differentially expressed proteins involved in transport and metabolism of amino acids, carbohydrates, coenzymes, and secondary metabolites consistently increased in the mycelium of both organisms (Fig. 4). The greater representation of proteins involved in carbohydrate transport and metabolism is not surprising considering that the mycelium is the vegetative growth life stage in which the organism utilizes nutrients from its environment. When Phytophthora grows necrotrophically inside the plant tissue, the environment in which the mycelium is present is rich in carbohydrates from the plant host, and utilization of these molecules is advantageous for the pathogen. Although most proteins in this category are associated with carbohydrate transport and glycolysis (catabolism of glucose for energy production), proteins in the other up-regulated mycelium functional categories are associated with biosynthesis. Thus, it appears that Phytophthora mycelium utilizes external glucose as an energy source to drive biosynthesis of amino acids and other small molecules necessary for vegetative growth.
Other classes of proteins up-regulated in the mycelium life stage included transporters, permeases, various lyases, kinases, and oxidoreductases (supplemental Tables S8 and S9). 26 orthologous proteins were up-regulated in the mycelia of both organisms and were identified as candidates for involvement specifically in vegetative growth (Fig. 3 and supplemental Table S10). One such pair includes P. sojae 132631 and P. ramorum 75997 (Table I). These proteins are coproporphyrinogen-III oxidases, an enzyme class participating in the process of heme biosynthesis (65). Heme and its derivatives are cofactors of different enzymes in different organisms, including cytochromes in the respiratory chains. In yeast, the rate-limiting coproporphyrinogen oxidase is transcriptionally induced under low oxygen conditions (66, 67), thus increasing heme production and possibly enabling improved respiration. Although it is unclear whether similar regulation of this enzyme occurs in Phytophthora, it is interesting to speculate that up-regulation of this enzyme during growth of the mycelium through the plant tissue, where oxygen levels are low, allows survival of the pathogen inside the plant tissue.
Other orthologous protein pairs up-regulated in mycelium are P. sojae 144346 and P. ramorum 85749 as well as P. sojae 145567 and P. ramorum 87858 (Table I). A close examination of the genomic sequence suggested that these were misannotated gene models encoding a single P. ramorum protein and a single P. sojae protein. These are annexin-like proteins, a family of eukaryotic Ca2+-dependent phospholipid-binding proteins involved in diverse functions such as exocytosis, membrane fusion, phospholipase inhibition, and Ca2+ channel regulation (68–70). P. ramorum 87858 has been identified previously as a mycelial cell wall-associated protein (71). Although the exact function of these Phytophthora annexins is unknown, they may be important for mycelial growth through the host tissue.
A final example of candidates that might be important for vegetative mycelium growth includes two orthologous protein pairs: P. sojae 127024 and P. ramorum 77742 (Table I) and P. sojae 127026 and P. ramorum 77744. These proteins contain catalytic domains similar to phospholipase D (PLD). PLDs are enzymes that hydrolyze the terminal phosphodiester bond of phospholipids into free choline and phosphatidic acid, a compound heavily involved in signal transduction (72). Phytophthora PLDs have unusual domain organization and composition as compared with other known eukaryotic PLDs and are thought to participate in non-typical biochemical pathways (73). The up-regulation of these proteins in the Phytophthora mycelium may facilitate signal transduction or other biochemical processes important for growth inside the plant tissue.
Identification of Candidate Proteins Involved in Host Specificity—
The host ranges of P. sojae and P. ramorum are very different. Although P. ramorum can infect a broad range of plant hosts, including many varieties of trees and shrubs, P. sojae can only infect certain varieties of soybean (Glycine max). The ability of P. sojae and P. ramorum to infect different hosts must be represented in their proteomic makeup. Therefore, the proteomes of the same life stage from P. sojae and P. ramorum were compared to identify candidate proteins involved in host range specificity. Orthologous proteins that did not follow a similar expression pattern and proteins with no orthologs that were expressed in one organism can be considered as such candidates. The list of proteins following these criteria was large (Fig. 6 and supplemental Tables S3, S4, and S11). In addition to proteins involved in host specificity, this list likely contained proteins that account for other differences between P. sojae and P. ramorum, such as adaptation to growth environment (below ground versus foliar), sexuality (self-fertile versus outcrossing), and different metabolic requirements. We therefore examined the list manually for proteins whose predicted function suggests involvement in host specificity.
Fig. 6.
Identification of candidate proteins for involvement in narrow versus broad host range capabilities. The total identified proteome (union of two MudPIT experiments) of each P. ramorum life stage was compared with the proteome of the same life stage from P. sojae (top and bottom overlapping circles). Overlapping regions represent orthologous proteins found in both, whereas non-overlapping regions represent proteins with no orthologs and proteins whose orthologous counterparts were not identified in the other organism. Also orthologous proteins that were identified in both organisms but followed a different expression pattern with respect to the life stages (middle overlapping circles) were also identified as candidates for host range capabilities. Pr, P. ramorum; Ps, P. sojae.
Candidate P. sojae Proteins Involved in Soybean Specificity (Narrow Host Range)—
Many of the proteins that were identified as candidates specifically involved in P. sojae host range were annotated as protein or carbohydrate hydrolases. One such example is P. sojae protein ID 127028 (Table I) that was found to be up-regulated in the germinating cyst. No P. ramorum ortholog was identified for this protein, and P. ramorum proteins that shared sequence similarity with it were undetected in both life stages of P. ramorum. This protein belongs to the glycosyl hydrolase family 81 (β-1,3-glucanases) and shares sequence similarity with glycosyl hydrolases from different fungi. Glycosyl hydrolases are enzymes that catalyze hydrolysis of the glycosidic bond between carbohydrates or between a carbohydrate and a non-carbohydrate molecule (74), and this protein may be involved in degradation of specific soybean polysaccharides or glycoproteins.
A second interesting group of proteins that may be specific to soybean infection includes 31 P. sojae proteins with high sequence similarity to the crinkling- and necrosis-inducing proteins CRN2, CRN3, CRN5, CRN7, and CRN13 from P. infestans. Nearly all of these P. sojae proteins were found to be up-regulated in the germinating cyst life stage, although some less than 5-fold (supplemental Table S12; two examples are presented in Table I), and had no P. ramorum orthologs. The CRN family of proteins is unique to Phytophthora (75). Although their function is not precisely defined, members of this family are secreted proteins that have been suggested to play an important part in the interaction with the host plant (38). Both resistant and susceptible host plants showed a necrotic response as well as other defense responses to virally transduced P. infestans crn2 (75). It is possible that these P. sojae proteins are required for successful infection initiation of soybean but not for P. ramorum hosts.
A final example of a protein that may contribute to successful infection of soybean is P. sojae 140694. This is an extracellular protein that was highly up-regulated in the mycelium (Table I). Although multiple homologous proteins are predicted in P. ramorum, most of them were undetected, none were up-regulated in the mycelium life stage, and some were slightly up-regulated in the germinating cyst. This P. sojae protein shares sequence and domain similarity with the cellulose-binding elicitor lectin (CBEL) group of Phytophthora proteins. CBELs are glycoproteins that are localized to the Phytophthora cell surface and elicit hypersensitive response-like necrosis and defense gene expression in the host plant (76, 77). Functionally they are implicated in adhesion and cell wall structure as well as in sensing exogenous cellulose (78). The unique expression pattern of P. sojae 140694 may represent a specific adaptation to soybean invasion or evasion from its early defense responses. Another possibility is that the broad host range P. ramorum has a more elaborate regulation of its homologous CBEL proteins and expresses them only in the presence of a specific host.
Candidate P. ramorum Proteins Involved in Broad Host Range Capability—
Proteins uniquely expressed in P. ramorum may contribute to its ability to infect a broad range of hosts. As was the case for P. sojae, the identified candidates included peptidases and other hydrolases, oxidoreductases, and kinases. Another family of P. ramorum proteins that may be involved in broad host range infection ability includes protein IDs 78733, 78734, 78735, 78744, 86077, and 86078. All of these proteins were found to be up-regulated in P. ramorum mycelium (example in Table I). Although four of the six proteins had orthologs in P. sojae, these orthologs as well as non-orthologous proteins with sequence similarity were not detected in both life stages of P. sojae. Although annotated as unknowns, these P. ramorum proteins show high similarity to a predicted nucleoside-diphosphate-sugar epimerase from the plant-pathogenic ascomycete fungus Gibberella zeae (anamorph Fusarium graminearum), the causative agent of head blight of wheat (79). Epimerases are ubiquitous enzymes that change the stereochemistry of carbohydrate moieties and are involved in many cellular processes (80). In bacteria epimerases are involved in production of complex carbohydrate polymers that, when incorporated into the cell wall or envelop, protect the cell from its host's immune responses (80). Although some epimerases are involved in vital cellular processes, such as the Calvin cycle in plants (80), it is possible that because their P. sojae counterparts were not detected these P. ramorum proteins are epimerases involved in production of carbohydrates that protect P. ramorum from its different plant hosts or allow utilization of a broader range of host sugars.
As a final example, protein 76706 was found to be up-regulated in the mycelium of P. ramorum, and no direct ortholog to it was identified in P. sojae (Table I). Although multiple P. sojae proteins showed high sequence similarity to it, none were expressed at a significant level. This P. ramorum protein was annotated as a sugar transporter of the major facilitator superfamily. Proteins of this family are integral membrane proteins capable of transporting a wide variety of substrates, including simple sugars, oligosaccharides, inositols, drugs, amino acids, nucleosides, organophosphate esters, Krebs cycle metabolites, and many different organic and inorganic ions (81). One possibility is that uptake of certain molecules present in the protoplast of the host plant by the P. ramorum mycelium assists survival and growth of the pathogen inside the plant tissue.
Conclusions—
A novel proteomics strategy for identification of candidate proteins involved in different cellular processes was applied to two life stages of two Phytophthora species. 91 orthologous protein pairs that followed similar expression patterns in P. ramorum and P. sojae were identified as candidates for early infection or for vegetative growth. Many species-specific proteins were identified, some of which were suggested as candidates for broad or narrow host range capability. These include peptidases and other hydrolases, transporters, and proteins involved in signal transduction mechanisms. Global analysis of the germinating cyst proteome from both species showed increased production of proteins involved in lipid transport and metabolism, cytoskeleton, and protein synthesis. Global analysis of the mycelium life stage showed increased production of proteins involved in transport and metabolism of many molecules, including amino acids, carbohydrates, coenzymes, and secondary metabolites.
Based on these finding we propose a model in which germinating cysts of Phytophthora catabolize lipid reserves through the β-oxidation pathway as an energy source (Fig. 7A). This energy is used to gear up protein production in the transition from a cyst to mycelium as well as for the production of the germ tube and initiating infection. During the process of germination, structural cytoskeleton proteins are up-regulated for construction of the germ tube, and proteins involved in microtubule-based movement are up-regulated for shuttling of molecules required for successful infection to the terminal end of the germ tube where interaction with the plant host takes place. The germinating cyst adheres to the plant host through the expression of secreted and membrane structural proteins, including laminin-type EGF-like domain-containing proteins. Combating host defenses is achieved through expression of enzymes that manipulate host defenses or degrade host proteins and carbohydrates.
Fig. 7.
Proposed model for Phytophthora germinating cyst and mycelium metabolic regulation. Based on the proteins that were found to be up-regulated in their respective life stages, it is proposed that the germinating cysts catabolize internal lipid reserves and use this energy for extensive protein synthesis including cytoskeleton and motor proteins required for generation of the germ tube (A). Adhesion proteins are also up-regulated and mediate adhesion of the germinating cyst to the plant. Once inside the host, Phytophthora grows vegetatively as mycelium where host sugars are transported and utilized as an energy source for glycolysis (B). Energy derived by this process is used to drive synthesis of many small molecules, including amino acids, coenzymes, and secondary metabolites.
Once inside the host tissue, the pathogen grows vegetatively as mycelium. At this life stage, the pathogen absorbs its nutrients from the degraded tissue of its plant host. Specifically the host nutrients are utilized as fuel to drive glycolysis, and the energy that is produced by this process is utilized to drive synthesis of amino acids and other small molecules necessary for vegetative growth (Fig. 7B).
Each Phytophthora species expresses a unique set of proteins during infection. These include proteases and other hydrolyzes as well as proteins that are involved in protein-protein interaction and in signal transduction. P. sojae, which has a narrow host range and can only infect soybean, uniquely expresses a group of crinkling- and necrosis-inducing proteins during cyst germination that may contribute to its specificity for soybean. P. ramorum, which has a broad host range, uniquely expresses a group of proteins involved in sugar transport and modification during vegetative growth that may aid in infection of its different hosts. The hypothetical role of targets identified in this study may be examined in the future using molecular tools available for Phytophthora manipulation, including RNA interference, targeted induced local lesions in genomes (TILLING), transformation, and classical genetics. Indeed our future study will focus on further characterization of early infection and species-specific targets identified in this study.
With few exceptions, our proteomics study agrees with or complements results from several transcriptomics studies carried out in Phytophthora (82–85). Recent studies of P. sojae (83, 85) and Phytophthora parasitica (82) identified multiple expressed sequence tags representing genes involved in glycolysis in mycelium or in planta. Contrary to our results, differential hybridization analysis of cDNA libraries from Phytophthora nicotianae identified glycolytic genes to be up-regulated in the germinating cyst (84). However, in accordance with our finding, the same study did show up-regulation of genes encoding proteins involved in protein biosynthesis, cell wall biogenesis, and adhesion in the germinating cyst. Using two-dimensional gel electrophoresis and real time RT-PCR in P. infestans, Grenville-Briggs et al. (86) identified five genes involved in amino acid biosynthesis to be up-regulated in both appressorium-forming germinating cyst and potato infection. With one exception (threonine synthase), homologs of all of these genes in P. sojae and P. ramorum were found to be somewhat up-regulated in mycelium in this study. Torto et al. (85) have also identified many expressed sequence tags representing P. sojae genes potentially involved in pathogenicity, such as hydrolases, elicitins, CRNs, and RXLR proteins. The proteomics study presented here not only corroborates the presence of these gene products but also suggests their temporal expression. Any discrepancy between our proteomics identification and previous transcriptomics studies might be explained by sampling bias of each technique, biological variation of different species, different growth conditions or sample preparation, or a true lack of correlation between the mRNA level and protein level in the cell.
Currently the genomes of Phytophthora capsici and P. infestans are being sequenced. Both organisms have host ranges that are very different from those of P. sojae and P. ramorum. Our future plans are to expand the cross-species proteomics analysis to include these newly sequenced Phytophthora species. This should prove useful for testing whether the proteins identified in this study are life stage- or host range-specific and will help refine the model described above. Additionally once a susceptible host is fully sequenced, a proteomics study of Phytophthora as it infects the plant will allow examination of our infection model. And finally, the strategy for cross-species comparative analysis outlined here provides a powerful strategy for investigating biological processes in closely related organisms.
Supplementary Material
Footnotes
Published, MCP Papers in Press, March 3, 2008, DOI 10.1074/mcp.M700431-MCP200
The abbreviations used are: MudPIT, multidimensional protein identification technology; NSAF, normalized spectral abundance factor; GO, Gene Ontology; KOG, Eukaryotic Orthologous Groups; ID, identity; BLAST, Basic Local Alignment Search Tool; EGF, epidermal growth factor; SIG, sexually induced gene; PLD, phospholipase D; CRN, crinkling- and necrosis-inducing protein; CBEL, cellulose-binding elicitor lectin.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Erwin, D. C., and Ribeiro, O. K. ( 1996) Phytophthora Diseases Worldwide, The American Phytopathological Society, St. Paul, MN
- 2.Rizzo, D. M., Garbelotto, M., Davidson, J. M., Slaughter, G. W., and Koike, S. T. ( 2002) Phytophthora ramorum as the cause of extensive mortality of Quercus spp., and Lithocarpus densiflorus in California. Plant Dis. 86, 205–214 [DOI] [PubMed] [Google Scholar]
- 3.Werres, S., Marwitz, R., Man in't Veld, W. A., De Cock, A. W. A. M., Bonants, P. J. M., De Weerdt, M., Themann, K., Ilieva, E., and Baayen, R. P. ( 2001) Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycol. Res. 10, 1166–1175 [Google Scholar]
- 4.Rizzo, D. M., Garbelotto, M., and Hansen, E. M. ( 2005) Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annu. Rev. Phytopathol. 43, 309–335 [DOI] [PubMed] [Google Scholar]
- 5.Tyler, B. M., Tripathy, S., Zhang, X., Dehal, P., Jiang, R. H., Aerts, A., Arredondo, F. D., Baxter, L., Bensasson, D., Beynon, J. L., Chapman, J., Damasceno, C. M., Dorrance, A. E., Dou, D., Dickerman, A. W., Dubchak, I. L., Garbelotto, M., Gijzen, M., Gordon, S. G., Govers, F., Grunwald, N. J., Huang, W., Ivors, K. L., Jones, R. W., Kamoun, S., Krampis, K., Lamour, K. H., Lee, M. K., McDonald, W. H., Medina, M., Meijer, H. J., Nordberg, E. K., Maclean, D. J., Ospina-Giraldo, M. D., Morris, P. F., Phuntumart, V., Putnam, N. H., Rash, S., Rose, J. K., Sakihama, Y., Salamov, A. A., Savidor, A., Scheuring, C. F., Smith, B. M., Sobral, B. W., Terry, A., Torto-Alalibo, T. A., Win, J., Xu, Z., Zhang, H., Grigoriev, I. V., Rokhsar, D. S., and Boore, J. L. ( 2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313, 1261–1266 [DOI] [PubMed] [Google Scholar]
- 6.Jiang, R. H., Tyler, B. M., and Govers, F. ( 2006) Comparative analysis of Phytophthora genes encoding secreted proteins reveals conserved synteny and lineage-specific gene duplications and deletions. Mol. Plant-Microbe Interact. 19, 1311–1321 [DOI] [PubMed] [Google Scholar]
- 7.Washburn, M. P., Wolters, D., and Yates, J. R., III ( 2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19, 242–247 [DOI] [PubMed] [Google Scholar]
- 8.Cagney, G., Park, S., Chung, C., Tong, B., O'Dushlaine, C., Shields, D. C., and Emili, A. ( 2005) Human tissue profiling with multidimensional protein identification technology. J. Proteome Res. 4, 1757–1767 [DOI] [PubMed] [Google Scholar]
- 9.Skipp, P., Robinson, J., O'Connor, C. D., and Clarke, I. N. ( 2005) Shotgun proteomic analysis of Chlamydia trachomatis. Proteomics 5, 1558–1573 [DOI] [PubMed] [Google Scholar]
- 10.Vitali, B., Wasinger, V., Brigidi, P., and Guilhaus, M. ( 2005) A proteomic view of Bifidobacterium infantis generated by multi-dimensional chromatography coupled with tandem mass spectrometry. Proteomics 5, 1859–1867 [DOI] [PubMed] [Google Scholar]
- 11.Brown, S. D., Thompson, M. R., Verberkmoes, N. C., Chourey, K., Shah, M., Zhou, J., Hettich, R. L., and Thompson, D. K. ( 2006) Molecular dynamics of the Shewanella oneidensis response to chromate stress. Mol. Cell. Proteomics 5, 1054–1071 [DOI] [PubMed] [Google Scholar]
- 12.Chu, D. S., Liu, H., Nix, P., Wu, T. F., Ralston, E. J., Yates, J. R., III, and Meyer, B. J. ( 2006) Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature 443, 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verberkmoes, N. C., Shah, M. B., Lankford, P. K., Pelletier, D. A., Strader, M. B., Tabb, D. L., McDonald, W. H., Barton, J. W., Hurst, G. B., Hauser, L., Davison, B. H., Beatty, J. T., Harwood, C. S., Tabita, F. R., Hettich, R. L., and Larimer, F. W. ( 2006) Determination and comparison of the baseline proteomes of the versatile microbe Rhodopseudomonas palustris under its major metabolic states. J. Proteome Res. 5, 287–298 [DOI] [PubMed] [Google Scholar]
- 14.Florens, L., Washburn, M. P., Raine, J. D., Anthony, R. M., Grainger, M., Haynes, J. D., Moch, J. K., Muster, N., Sacci, J. B., Tabb, D. L., Witney, A. A., Wolters, D., Wu, Y., Gardner, M. J., Holder, A. A., Sinden, R. E., Yates, J. R., and Carucci, D. J. ( 2002) A proteomic view of the Plasmodium falciparum life cycle. Nature 419, 520–526 [DOI] [PubMed] [Google Scholar]
- 15.Leifso, K., Cohen-Freue, G., Dogra, N., Murray, A., and McMaster, W. R. ( 2007) Genomic and proteomic expression analysis of Leishmania promastigote and amastigote life stages: the Leishmania genome is constitutively expressed. Mol. Biochem. Parasitol 152, 35–46 [DOI] [PubMed] [Google Scholar]
- 16.Savidor, A., Donahoo, R. S., Hurtado-Gonzales, O., Verberkmoes, N. C., Shah, M. B., Lamour, K. H., and McDonald, W. H. ( 2006) Expressed peptide tags: an additional layer of data for genome annotation. J. Proteome Res. 5, 3048–3058 [DOI] [PubMed] [Google Scholar]
- 17.McDonald, W. H., Tabb, D. L., Sadygov, R. G., MacCoss, M. J., Venable, J., Graumann, J., Johnson, J. R., Cociorva, D., and Yates, J. R. ( 2004) MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom. 18, 2162–2168 [DOI] [PubMed] [Google Scholar]
- 18.Narasimhan, C., Tabb, D. L., Verberkmoes, N. C., Thompson, M. R., Hettich, R. L., and Uberbacher, E. C. ( 2005) MASPIC: intensity-based tandem mass spectrometry scoring scheme that improves peptide identification at high confidence. Anal. Chem. 77, 7581–7593 [DOI] [PubMed] [Google Scholar]
- 19.Tabb, D. L., Narasimhan, C., Strader, M. B., and Hettich, R. L. ( 2005) DBDigger: reorganized proteomic database identification that improves flexibility and speed. Anal. Chem. 77, 2464–2474 [DOI] [PubMed] [Google Scholar]
- 20.Tabb, D. L., McDonald, W. H., and Yates, J. R., III ( 2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabb, D. L., Shah, M. B., Strader, M. B., Connelly, H. M., Hettich, R. L., and Hurst, G. B. ( 2006) Determination of peptide and protein ion charge states by Fourier transformation of isotope-resolved mass spectra. J. Am. Soc. Mass Spectrom. 17, 903–915 [DOI] [PubMed] [Google Scholar]
- 22.Florens, L., Carozza, M. J., Swanson, S. K., Fournier, M., Coleman, M. K., Workman, J. L., and Washburn, M. P. ( 2006) Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40, 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benov, L., and Fridovich, I. ( 1995) Superoxide dismutase protects against aerobic heat shock in Escherichia coli. J. Bacteriol. 177, 3344–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fridovich, I. ( 1995) Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64, 97–112 [DOI] [PubMed] [Google Scholar]
- 25.Lanfranco, L., Novero, M., and Bonfante, P. ( 2005) The mycorrhizal fungus Gigaspora margarita possesses a CuZn superoxide dismutase that is up-regulated during symbiosis with legume hosts. Plant Physiol. 137, 1319–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Brugger, A., Lamotte, O., Vandelle, E., Bourque, S., Lecourieux, D., Poinssot, B., Wendehenne, D., and Pugin, A. ( 2006) Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 19, 711–724 [DOI] [PubMed] [Google Scholar]
- 27.Apostol, I., Heinstein, P. F., and Low, P. S. ( 1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 90, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennings, D. B., Ehrenshaft, M., Pharr, D. M., and Williamson, J. D. ( 1998) Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. U. S. A. 95, 15129–15133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller, T., Damude, H. G., Werner, D., Doerner, P., Dixon, R. A., and Lamb, C. ( 1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Groote, M. A., Ochsner, U. A., Shiloh, M. U., Nathan, C., McCord, J. M., Dinauer, M. C., Libby, S. J., Vazquez-Torres, A., Xu, Y., and Fang, F. C. ( 1997) Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 94, 13997–14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandell, G. L. ( 1975) Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal-leukocyte interaction. J. Clin. Investig. 55, 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narasipura, S. D., Ault, J. G., Behr, M. J., Chaturvedi, V., and Chaturvedi, S. ( 2003) Characterization of Cu, Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Mol. Microbiol. 47, 1681–1694 [DOI] [PubMed] [Google Scholar]
- 33.Birch, P. R., Rehmany, A. P., Pritchard, L., Kamoun, S., and Beynon, J. L. ( 2006) Trafficking arms: oomycete effectors enter host plant cells. Trends Microbiol. 14, 8–11 [DOI] [PubMed] [Google Scholar]
- 34.Ellis, J., Catanzariti, A. M., and Dodds, P. ( 2006) The problem of how fungal and oomycete avirulence proteins enter plant cells. Trends Plant Sci. 11, 61–63 [DOI] [PubMed] [Google Scholar]
- 35.Rehmany, A. P., Gordon, A., Rose, L. E., Allen, R. L., Armstrong, M. R., Whisson, S. C., Kamoun, S., Tyler, B. M., Birch, P. R., and Beynon, J. L. ( 2005) Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell 17, 1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, H., Sadygov, R. G., and Yates, J. R., III ( 2004) A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193–4201 [DOI] [PubMed] [Google Scholar]
- 37.Kunau, W. H., Dommes, V., and Schulz, H. ( 1995) β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 34, 267–342 [DOI] [PubMed] [Google Scholar]
- 38.Ebstrup, T., Saalbach, G., and Egsgaard, H. ( 2005) A proteomics study of in vitro cyst germination and appressoria formation in Phytophthora infestans. Proteomics 5, 2839–2848 [DOI] [PubMed] [Google Scholar]
- 39.Poirier, Y., Antonenkov, V. D., Glumoff, T., and Hiltunen, J. K. ( 2006) Peroxisomal β-oxidation—a metabolic pathway with multiple functions. Biochim. Biophys. Acta 1763, 1413–1426 [DOI] [PubMed] [Google Scholar]
- 40.Clark, M. C., Melanson, D. L., and Page, O. T. ( 1978) Purine metabolism and differential inhibition of spore germination in Phytophthora infestans. Can. J. Microbiol. 24, 1032–1038 [DOI] [PubMed] [Google Scholar]
- 41.Kramer, R., Freytag, S., and Schmelzer, E. ( 1997) In vitro formation of infection structures of Phytophthora infestans is associated with synthesis of stage specific polypeptides. Eur. J. Plant Pathol. 103, 43–53 [Google Scholar]
- 42.Penington, C. J., Iser, J. R., Grant, B. R., and Gayler, K. R. ( 1989) The role of RNA and protein synthesis in stimulated germination of the zoospores of the pathogenic fungus Phytophthora palmivora. Exp. Mycol. 13, 158–168 [Google Scholar]
- 43.Germain, V., Rylott, E. L., Larson, T. R., Sherson, S. M., Bechtold, N., Carde, J. P., Bryce, J. H., Graham, I. A., and Smith, S. M. ( 2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 28, 1–12 [DOI] [PubMed] [Google Scholar]
- 44.Pinfield-Wells, H., Rylott, E. L., Gilday, A. D., Graham, S., Job, K., Larson, T. R., and Graham, I. A. ( 2005) Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 43, 861–872 [DOI] [PubMed] [Google Scholar]
- 45.Hayashi, M., Toriyama, K., Kondo, M., and Nishimura, M. ( 1998) 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geopfert, S., and Poirier, Y. ( 2007) β-Oxidation in fatty acid degradation and beyond. Curr. Opin. Plant Biol. 10, 245–251 [DOI] [PubMed] [Google Scholar]
- 47.Footitt, S., Marquez, J., Schmuths, H., Baker, A., Theodoulou, F. L., and Holdsworth, M. ( 2006) Analysis of the role of COMATOSE and peroxisomal β-oxidation in the determination of germination potential in Arabidopsis. J. Exp. Bot. 57, 2805–2814 [DOI] [PubMed] [Google Scholar]
- 48.Penfield, S., Graham, S., and Graham, I. A. ( 2005) Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem. Soc. Trans. 33, 380–383 [DOI] [PubMed] [Google Scholar]
- 49.Dai, S., Chen, T., Chong, K., Xue, Y., Liu, S., and Wang, T. ( 2007) Proteomics identification of differentially expressed proteins associated with pollen germination and tube growth reveals characteristics of germinated Oryza sativa pollen. Mol. Cell. Proteomics 6, 207–230 [DOI] [PubMed] [Google Scholar]
- 50.Gallardo, K., Job, C., Groot, S. P., Puype, M., Demol, H., Vandekerckhove, J., and Job, D. ( 2001) Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 126, 835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.d'Enfert, C. ( 1997) Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 21, 163–172 [Google Scholar]
- 52.Dai, S., Li, L., Chen, T., Chong, K., Xue, Y., and Wang, T. ( 2006) Proteomic analyses of Oryza sativa mature pollen reveal novel proteins associated with pollen germination and tube growth. Proteomics 6, 2504–2529 [DOI] [PubMed] [Google Scholar]
- 53.Rajjou, L., Gallardo, K., Debeaujon, I., Vandekerckhove, J., Job, C., and Job, D. ( 2004) The effect of α-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol. 134, 1598–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell, L. D., and Bork, P. ( 1993) Epidermal growth factor-like modules. Curr. Opin. Struct. Biol. 3, 385–392 [Google Scholar]
- 55.Bork, P., Downing, A. K., Kieffer, B., and Campbell, I. D. ( 1996) Structure and distribution of modules in extracellular proteins. Q. Rev. Biophys. 29, 119–167 [DOI] [PubMed] [Google Scholar]
- 56.Beck, K., Hunter, I., and Engel, J. ( 1990) Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 4, 148–160 [DOI] [PubMed] [Google Scholar]
- 57.Engel, J. ( 1992) Laminins and other strange proteins. Biochemistry 31, 10643–10651 [DOI] [PubMed] [Google Scholar]
- 58.Hohenester, E., and Engel, J. ( 2002) Domain structure and organisation in extracellular matrix proteins. Matrix Biol. 21, 115–128 [DOI] [PubMed] [Google Scholar]
- 59.Liu, B. F., Ma, J., and Cui, F. Z. ( 2006) Regulation of charged groups and laminin patterns for selective neuronal adhesion. Colloids Surf. B Biointerfaces 53, 175–178 [DOI] [PubMed] [Google Scholar]
- 60.Armbrust, E. V. ( 1999) Identification of a new gene family expressed during the onset of sexual reproduction in the centric diatom Thalassiosira weissflogii. Appl. Environ. Microbiol. 65, 3121–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dodd, R. B., and Drickamer, K. ( 2001) Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology 11, 71R–79R [DOI] [PubMed] [Google Scholar]
- 62.Mishra, V., Ethayathulla, A. S., Sharma, R. S., Yadav, S., Krauspenhaar, R., Betzel, C., Babu, C. R., and Singh, T. P. ( 2004) Structure of a novel ribosome-inactivating protein from a hemi-parasitic plant inhabiting the northwestern Himalayas. Acta Crystallogr. Sect. D Biol. Crystallogr. 60, 2295–2304 [DOI] [PubMed] [Google Scholar]
- 63.Shimoi, H., Iimura, Y., Obata, T., and Tadenuma, M. ( 1992) Molecular structure of Rarobacter faecitabidus protease I. A yeast-lytic serine protease having mannose-binding activity. J. Biol. Chem. 267, 25189–25195 [PubMed] [Google Scholar]
- 64.al Daher, S., de Gasperi, R., Daniel, P., Hall, N., Warren, C. D., and Winchester, B. ( 1991) The substrate-specificity of human lysosomal α-d-mannosidase in relation to genetic alpha-mannosidosis. Biochem. J. 277, 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu, K., and Elliott, T. ( 1993) An oxygen-dependent coproporphyrinogen oxidase encoded by the hemF gene of Salmonella typhimurium. J. Bacteriol. 175, 4990–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zagorec, M., Buhler, J. M., Treich, I., Keng, T., Guarente, L., and Labbe-Bois, R. ( 1988) Isolation, sequence, and regulation by oxygen of the yeast HEM13 gene coding for coproporphyrinogen oxidase. J. Biol. Chem. 263, 9718–9724 [PubMed] [Google Scholar]
- 67.Zagorec, M., and Labbe-Bois, R. ( 1986) Negative control of yeast coproporphyrinogen oxidase synthesis by heme and oxygen. J. Biol. Chem. 261, 2506–2509 [PubMed] [Google Scholar]
- 68.Gerke, V., and Moss, S. E. ( 2002) Annexins: from structure to function. Physiol. Rev. 82, 331–371 [DOI] [PubMed] [Google Scholar]
- 69.Raynal, P., and Pollard, H. B. ( 1994) Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1197, 63–93 [DOI] [PubMed] [Google Scholar]
- 70.Romisch, J., and Paques, E. P. ( 1991) Annexins: calcium-binding proteins of multi-functional importance? Med. Microbiol. Immunol. 180, 109–126 [DOI] [PubMed] [Google Scholar]
- 71.Meijer, H. J., van de Vondervoort, P. J., Yin, Q. Y., de Koster, C. G., Klis, F. M., Govers, F., and de Groot, P. W. ( 2006) Identification of cell wall-associated proteins from Phytophthora ramorum. Mol. Plant-Microbe Interact. 19, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 72.Cazzolli, R., Shemon, A. N., Fang, M. Q., and Hughes, W. E. ( 2006) Phospholipid signalling through phospholipase D and phosphatidic acid. IUBMB Life 58, 457–461 [DOI] [PubMed] [Google Scholar]
- 73.Meijer, H. J., and Govers, F. ( 2006) Genomewide analysis of phospholipid signaling genes in Phytophthora spp.: novelties and a missing link. Mol. Plant-Microbe Interact. 19, 1337–1347 [DOI] [PubMed] [Google Scholar]
- 74.Henrissat, B., Callebaut, I., Fabrega, S., Lehn, P., Mornon, J. P., and Davies, G. ( 1995) Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc. Natl. Acad. Sci. U. S. A. 92, 7090–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torto, T. A., Li, S., Styer, A., Huitema, E., Testa, A., Gow, N. A., van West, P., and Kamoun, S. ( 2003) EST mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora. Genome Res. 13, 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mateos, F. V., Rickauer, M., and Esquerre-Tugaye, M. T. ( 1997) Cloning and characterization of a cDNA encoding an elicitor of Phytophthora parasitica var. nicotianae that shows cellulose-binding and lectin-like activities. Mol. Plant-Microbe Interact. 10, 1045–1053 [DOI] [PubMed] [Google Scholar]
- 77.Séjalon-Delmas, N., Villalba, F., Bottin, A., Rickauer, M., Dargent, R., and Esquerré-Tugayé, M. T. ( 1997) Purification, elicitor activity, and cell wall localisation of a glycoprotein from Phytophthora parasitica var. nicotianae, a fungal pathogen of tobacco. Phytopathology 87, 899–909 [DOI] [PubMed] [Google Scholar]
- 78.Gaulin, E., Jauneau, A., Villalba, F., Rickauer, M., Esquerre-Tugaye, M. T., and Bottin, A. ( 2002) The CBEL glycoprotein of Phytophthora parasitica var-nicotianae is involved in cell wall deposition and adhesion to cellulosic substrates. J. Cell Sci. 115, 4565–4575 [DOI] [PubMed] [Google Scholar]
- 79.Bai, G., and Shaner, G. ( 2004) Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 42, 135–161 [DOI] [PubMed] [Google Scholar]
- 80.Allard, S. T., Giraud, M. F., and Naismith, J. H. ( 2001) Epimerases: structure, function and mechanism. CMLS Cell. Mol. Life Sci. 58, 1650–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pao, S. S., Paulsen, I. T., and Saier, M. H., Jr. ( 1998) Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62, 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panabières, F., Amselem, J., Galiana, E., and Le Berre, J. Y. ( 2005) Gene identification in the oomycete pathogen Phytophthora parasitica during in vitro vegetative growth through expressed sequence tags. Fungal Genet. Biol. 42, 611–623 [DOI] [PubMed] [Google Scholar]
- 83.Qutob, D., Hraber, P. T., Sobral, B. W., and Gijzen, M. ( 2000) Comparative analysis of expressed sequences in Phytophthora sojae. Plant Physiol. 123, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shan, W., Marshall, J. S., and Hardham, A. R. ( 2004) Gene expression in germinated cysts of Phytophthora nicotianae. Mol. Plant Pathol. 5, 317–330 [DOI] [PubMed] [Google Scholar]
- 85.Torto-Alalibo, T. A., Tripathy, S., Smith, B. M., Arredondo, F. D., Zhou, L., Li, H., Chibucos, M. C., Qutob, D., Gijzen, M., Mao, C., Sobral, B. W., Waugh, M. E., Mitchell, T. K., Dean, R. A., and Tyler, B. M. ( 2007) Expressed sequence tags from Phytophthora sojae reveal genes specific to development and infection. Mol. Plant-Microbe Interact. 20, 781–793 [DOI] [PubMed] [Google Scholar]
- 86.Grenville-Briggs, L. J., Avrova, A. O., Bruce, C. R., Williams, A., Whisson, S. C., Birch, P. R., and van West, P. ( 2005) Elevated amino acid biosynthesis in Phytophthora infestans during appressorium formation and potato infection. Fungal Genet. Biol. 42, 244–256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.