Abstract
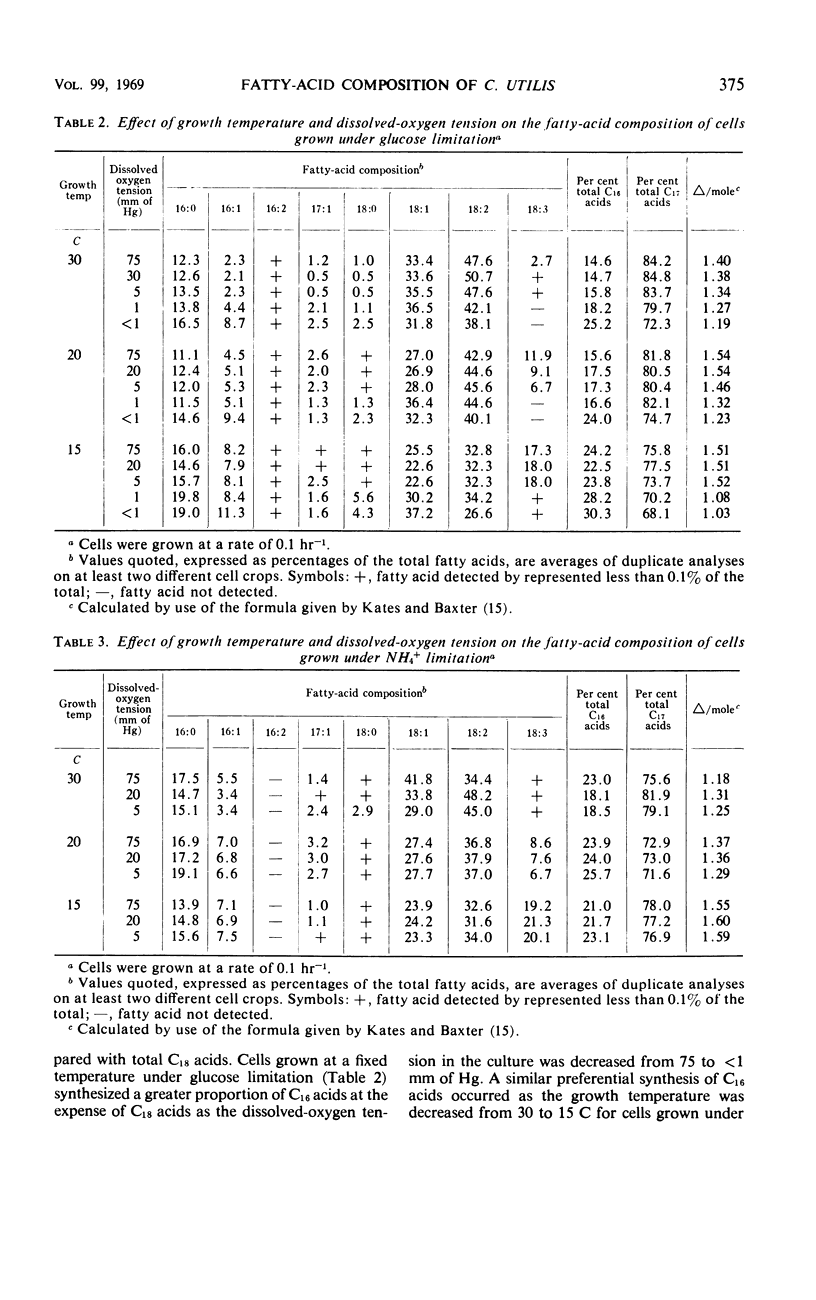
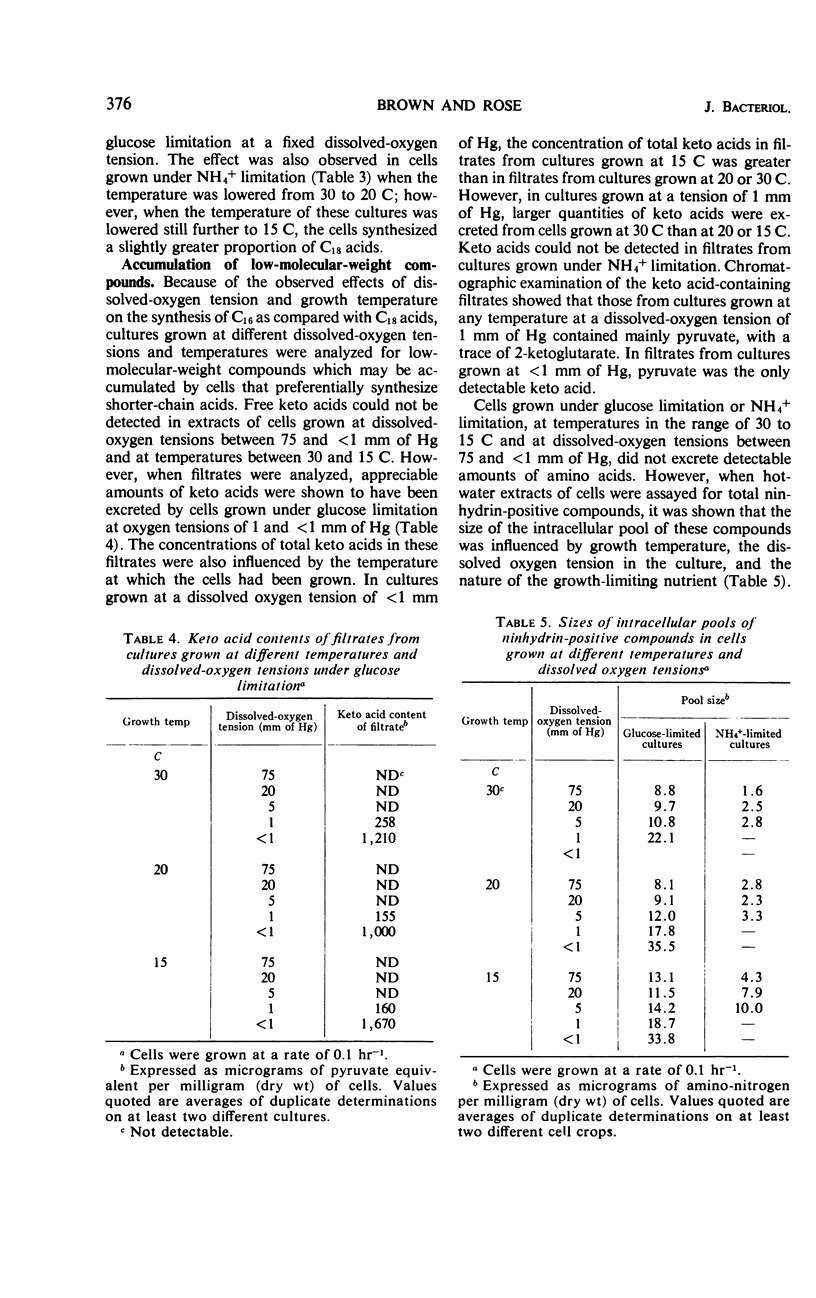
Analyses were made of the fatty-acid composition of Candida utilis NCYC 321 grown in a chemostat at a dilution rate (equal to growth rate) of 0.1 hr−1 and at temperatures in the range of 30 to 15 C and dissolved oxygen tensions between 75 and <1 mm of Hg. Cells grown under glucose limitation or NH4+ limitation contained mainly C16:0, C16:1, C18:0, C18:1, C18:2, and C18:3 acids as detected by gas-liquid chromatography of methyl esters of the acids from lipids extracted with chloroform-methanol. The relative proportions of these acids varied with the growth temperature and the dissolved-oxygen tension in the culture. A decrease in growth temperature from 30 to 20 C led to an increased synthesis of unsaturated acids in cells grown under either limitation at a fixed-oxygen tension in the range of 75 to 5 mm of Hg. In cultures with a dissolved-oxygen tension of 1 and <1 mm of Hg, a further decrease in temperature to 15 C caused an increased synthesis of unsaturated fatty acids. A decrease in dissolved-oxygen tension led to a diminished synthesis of unsaturated fatty acids in cells grown at a fixed temperature under either limitation. Cells grown at a fixed temperature under glucose limitation synthesized a greater proportion of C16 acids at the expense of C18 acids as the dissolved oxygen tension was decreased from 75 to <1 mm of Hg. A preferential synthesis of C16 acids also occurred as the growth temperature was decreased from 30 to 15 C in cells grown under glucose limitation at a fixed-oxygen tension. The same effect was observed in cells grown under NH4+ limitation when the temperature was lowered from 30 to 20 C; but when the temperature was decreased further to 15 C, the cells synthesized a slightly greater proportion of C18 acids. Synthesis of a large proportion of C16 acids was accompanied by an excretion of pyruvate, and occasionally traces of 2-ketoglutarate, and an increased intracellular accumulation of certain amino acids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- BLOOMFIELD D. K., BLOCH K. The formation of delta 9-unsaturated fatty acids. J Biol Chem. 1960 Feb;235:337–345. [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Effects of temperature on composition and cell volume of Candida utilis. J Bacteriol. 1969 Jan;97(1):261–270. doi: 10.1128/jb.97.1.261-272.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. S., Craig B. M. Lipids of Candida utilis: changes with growth. Can J Microbiol. 1966 Aug;12(4):775–785. doi: 10.1139/m66-105. [DOI] [PubMed] [Google Scholar]
- Farrell J., Rose A. Temperature effects on microorganisms. Annu Rev Microbiol. 1967;21:101–120. doi: 10.1146/annurev.mi.21.100167.000533. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. The effect of temperature on the formation of delta 5-unsaturated fatty acids by bacilli. Biochim Biophys Acta. 1967 Dec 5;144(3):701–703. doi: 10.1016/0005-2760(67)90065-3. [DOI] [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Kates M. Biosynthesis of lipids in microorganisms. Annu Rev Microbiol. 1966;20:13–44. doi: 10.1146/annurev.mi.20.100166.000305. [DOI] [PubMed] [Google Scholar]
- LIGHT R. J., LENNARZ W. J., BLOCH K. The metabolism of hydroxystearic acids in yeast. J Biol Chem. 1962 Jun;237:1793–1800. [PubMed] [Google Scholar]
- Longley R. P., Rose A. H., Knights B. A. Composition of the protoplast membrane from Saccharomyces cerevisiae. Biochem J. 1968 Jul;108(3):401–412. doi: 10.1042/bj1080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. G., Pirt S. J. Automatic control of dissolved oxygen concentration in stirred microbial cultures. J Gen Microbiol. 1966 Nov;45(2):289–302. doi: 10.1099/00221287-45-2-289. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE A. H., EVISON L. M. STUDIES ON THE BIOCHEMICAL BASIS OF THE MINIMUM TEMPERATURES FOR GROWTH OF CERTAIN PSYCHROPHILIC AND MESOPHILIC MICRO-ORGANISMS. J Gen Microbiol. 1965 Jan;38:131–141. doi: 10.1099/00221287-38-1-131. [DOI] [PubMed] [Google Scholar]



